Latest Competitive Analysis of Merck Sharp & Dohme Drug Pipeline
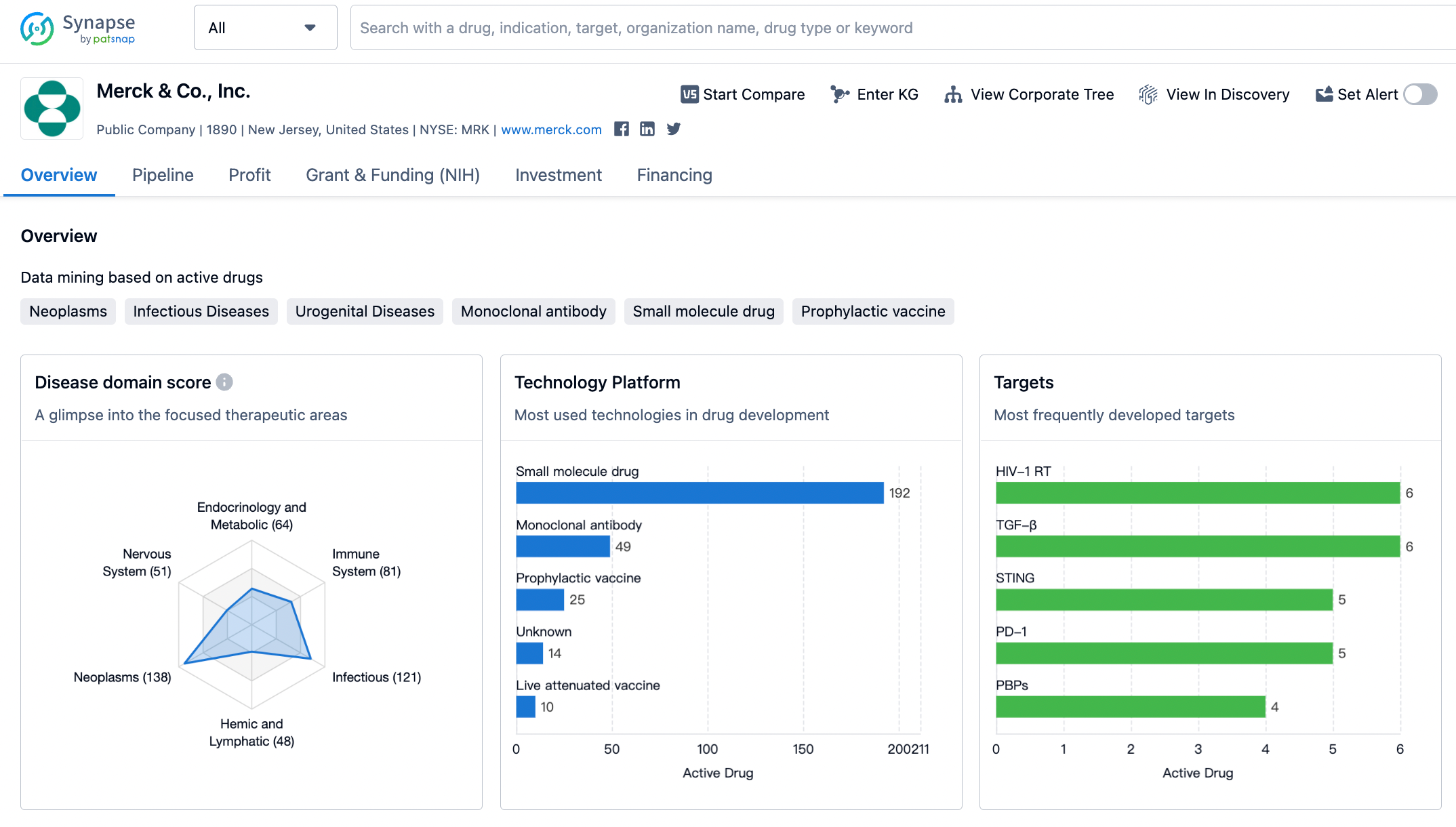
Merck's recent revenue performance remains steady, with the K drug continuing to grow and earning the title of "New Generation Drug King". Additionally, the HPV vaccine also continues to maintain its momentum. These two key products have managed to fill the gap left by the rapid shrinkage of Molnupiravir and the patent cliff of Sitagliptin. Despite a significant decline in profitability, the market value is still approaching $300 billion.
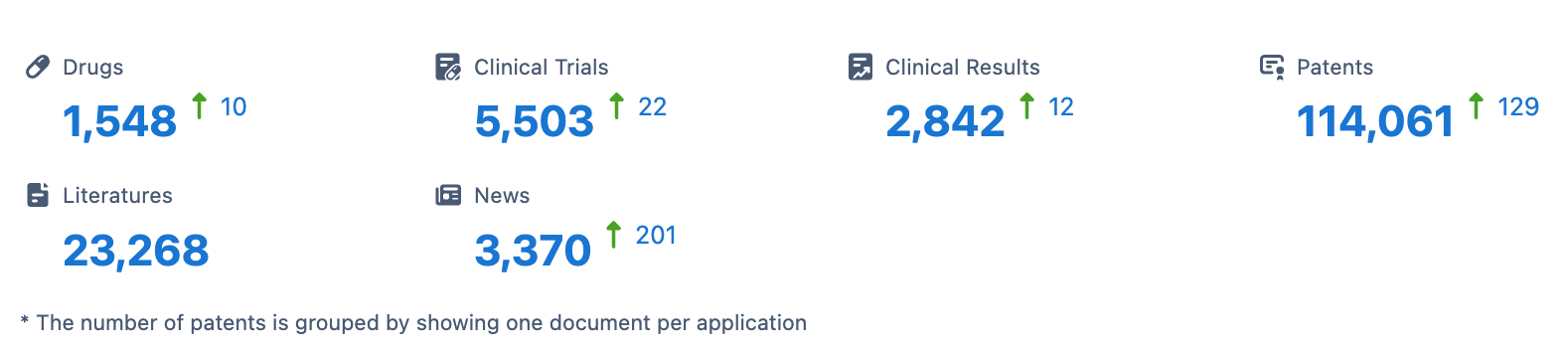
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Merck.
A significant portion of ongoing research and development still centers around various expanded applications and combination plans for the K drug.
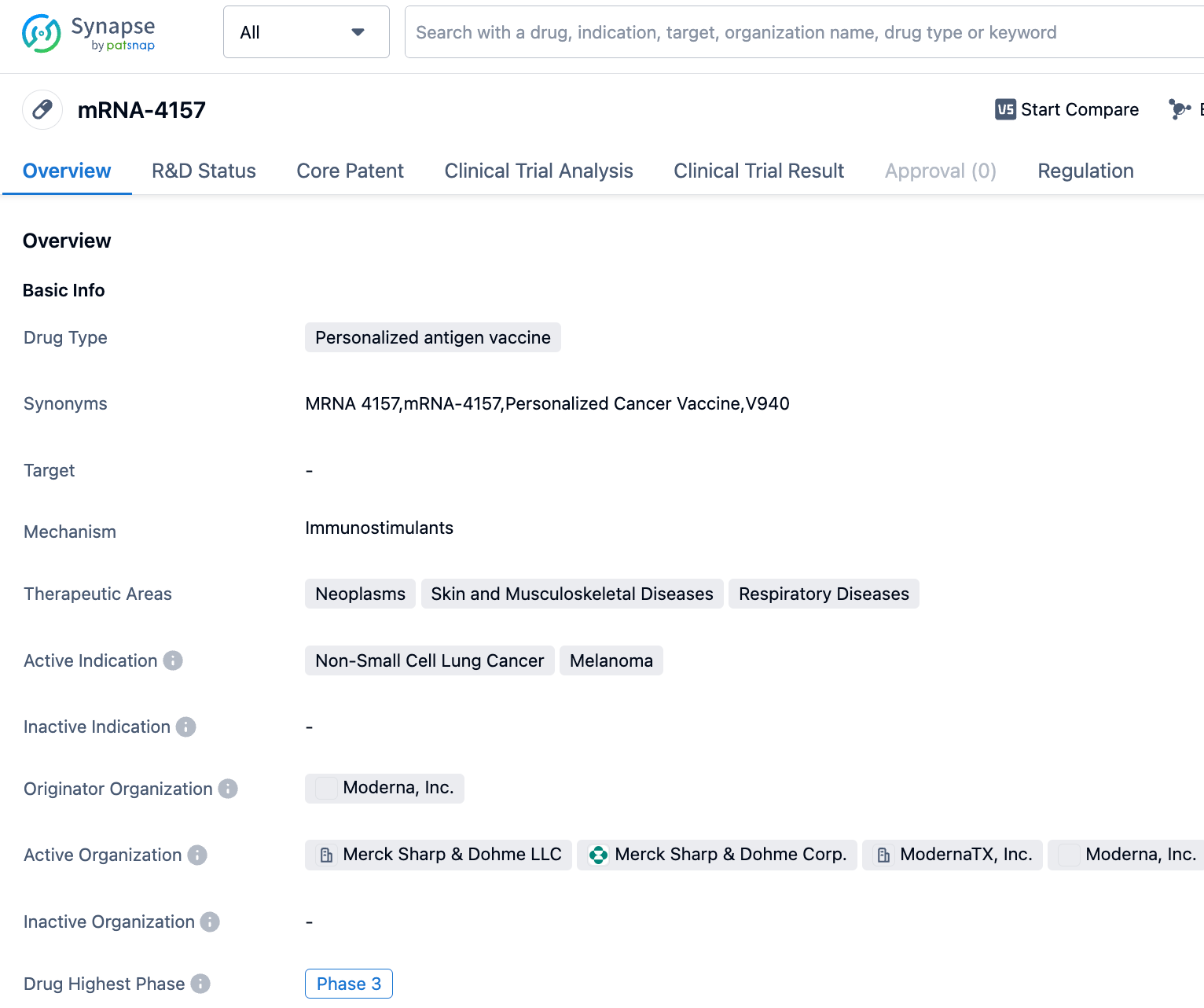
Noteworthy recent advancements include Phase III clinical results for the adjuvant/neoadjuvant treatment of stage II/IIIA/IIIB NSCLC, and Phase 2B clinical results for the mRNA-4157 cancer vaccine developed in partnership with Moderna for treating melanoma.
Cardiovascular pipeline developments include Phase III clinical data and NDA submission for the PAH drug Sotatercept, Phase 2A clinical results for the GLP-1/GCGR dual agonist Efinopegdutide in treating NAFLD, and Phase II clinical data for the oral PCSK9 drug MK-0616. Vaccine pipeline advancements include preliminary Phase III clinical results for the 21-valent pneumococcal vaccine V-116, and the acquisition of Prometheus to obtain the TL1A antibody.