Latest Competitive Analysis of Roche Drug Pipeline
The Swiss pharmaceutical giant Roche has seen sluggish growth in its oncology division, with almost no increase in four years, worsened further by the weakening of the diagnostic division due to the COVID-19 pandemic. Its market value has dropped from 300 billion to around 250 billion.
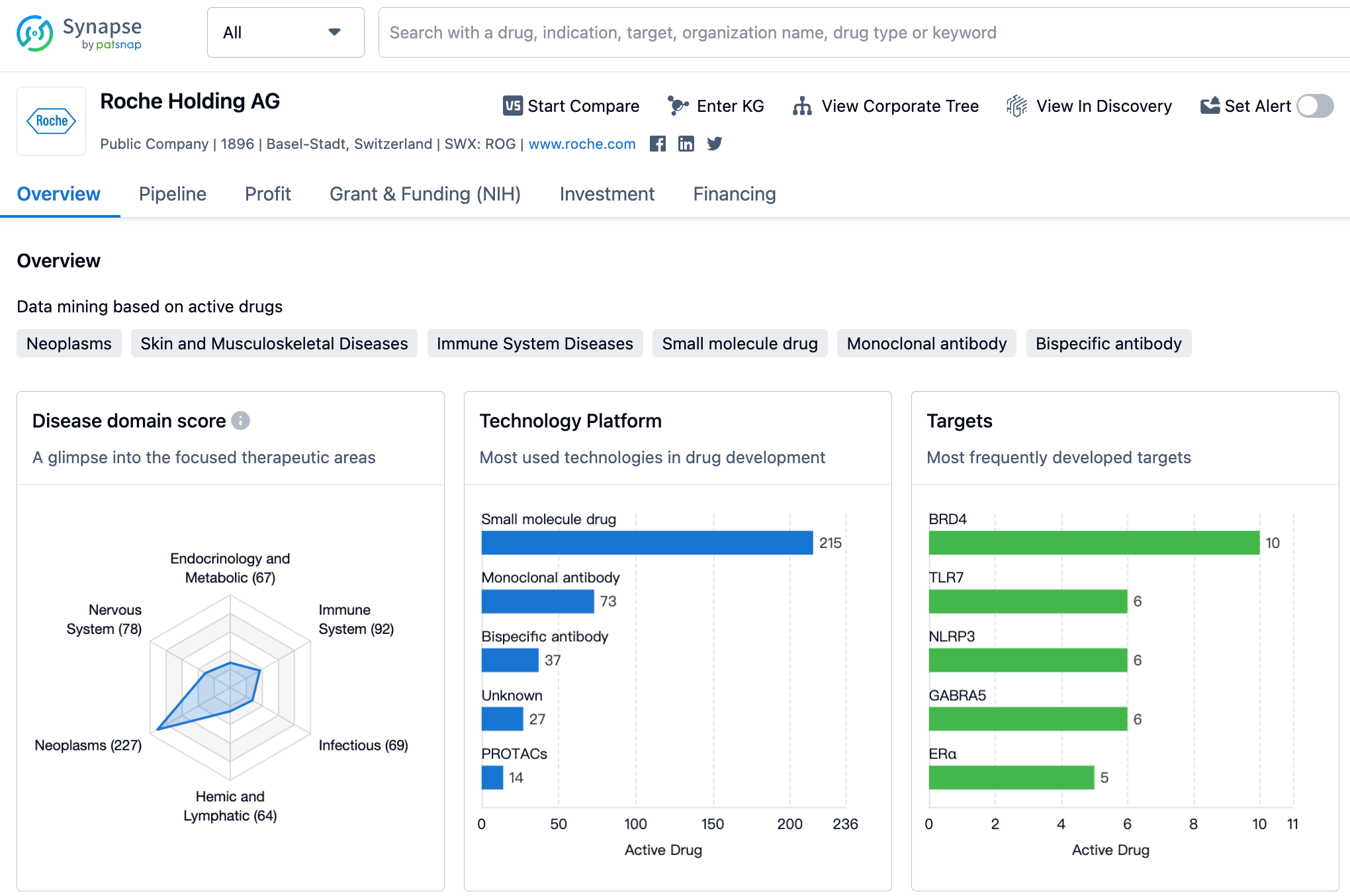
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Roche.
With popular drugs of the past epoch, such as Herceptin, Avastin, Mabthera, and Lucentis on the decline, Roche faces a massive patent cliff and a drought of big-name products. The uphill battle of the past few years has been challenging. Fortunately, new pillars have begun to emerge, including ophthalmology dual antibodies Faricimab, hemophilia dual antibodies Emicizumab, and the new generation of CD20 monoclonal antibody, Ocrelizumab. While not as phenomenal as their predecessors, these products have filled the gap with billion-dollar contributions.
In terms of research and development, after a nightmarish 2022, Roche has finally gained some ground. Most notably are the OS data of the TIGIT monoclonal antibody Tiragolumab in phase III NSCLC clinical trials and the latest results from the combined T+A regimen in phase III 1L HCC clinical. These triggered a resurgence of hope.
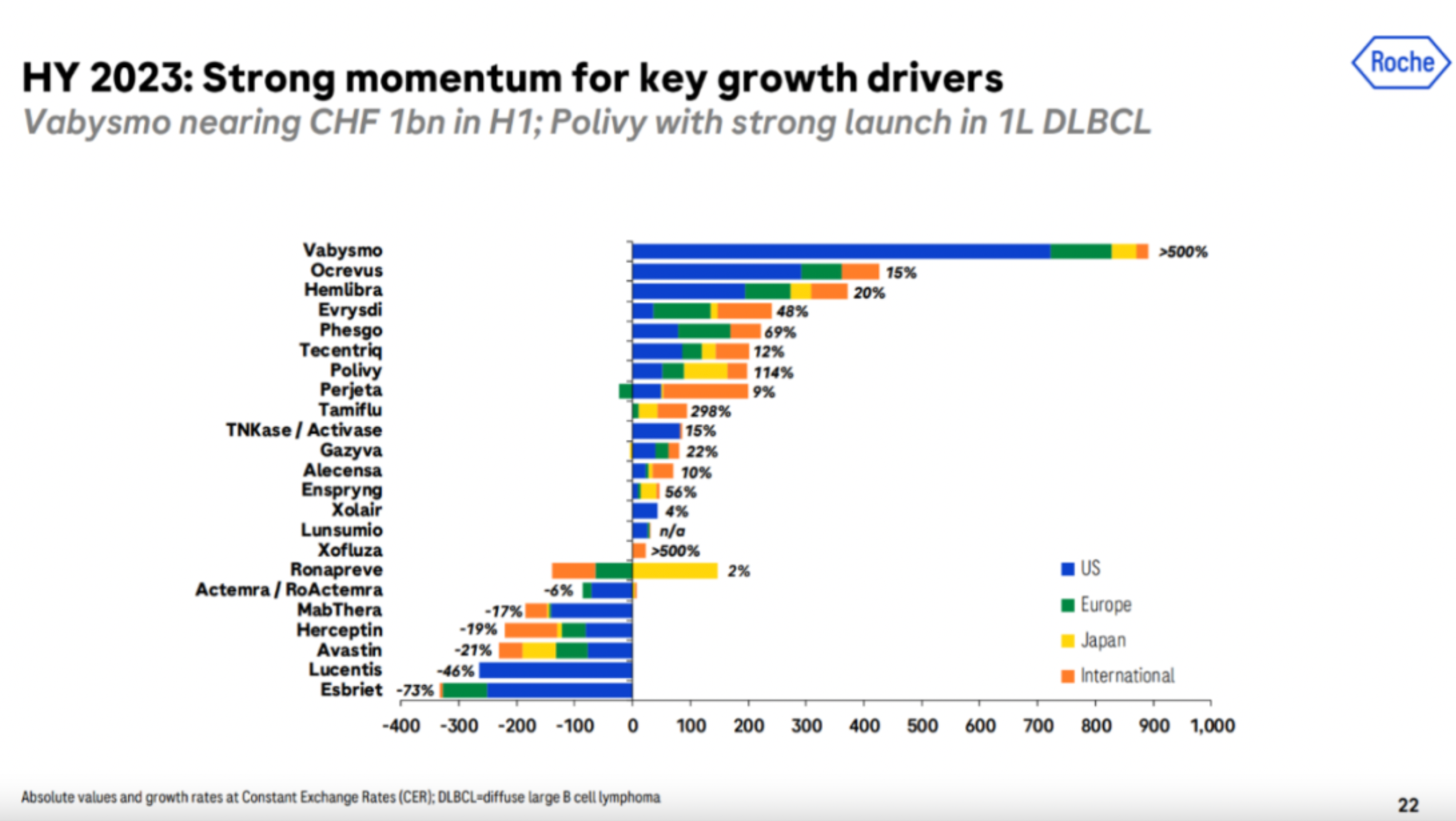
Additionally, the company's second CD20×CD3 dual antibodies Glofitamab and DMD gene therapy Elevidys, Polivy in combination with R-CHP approved for 1L DLBCL indications, Faricimab approved for RVO indications, C5 monoclonal antibody Crovalimab published 2 PNH phase III clinical results, and the new generation BTK inhibitor Fenebrutinib, etc., have all made significant progress. Roche has also introduced Zilebesiran, a siRNA drug targeting AGT from Alnylam.