LEO Pharma Inc. has confirmed that the FDA has endorsed Adbry® (tralokinumab-ldrm) to manage adolescents aged 12-17 with moderate to severe eczema
LEO Pharma Inc. has disclosed that the FDA has recently broadened the sanctioned use of Adbry (tralokinumab-ldrm) by authorizing its application for young individuals between the ages of 12 and 17 who are coping with a moderate to severe form of atopic dermatitis. This extended approval applies specifically to cases in which conventional topical treatments prove insufficient or are considered unsuitable.
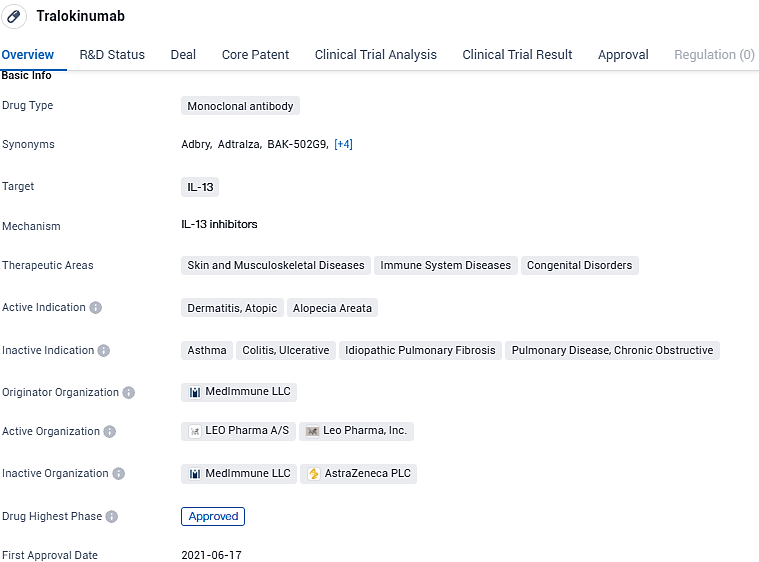
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Adbry® represents the singular biologic approved by the FDA that exclusively targets and neutralizes the interleukin (IL)-13 cytokine, a principal factor contributing to the clinical manifestations of Atopic Dermatitis (AD).
"Reaching this landmark achievement significantly propels our mission to profoundly influence the lives of those in dire need of new treatments. The availability of Adbry introduces an essential alternative for managing atopic dermatitis in this specific patient population," commented Brian Hilberdink, EVP and President of LEO Pharma's Region North America.
Expressing satisfaction with the progress, Brian Hilberdink further remarked, "It's gratifying for us to extend Adbry—an advanced, precision therapy—to both adults and children afflicted with AD in the United States. For the younger demographics, in particular, AD's severe implications can lead to social isolation. We take immense pride in our advancements and remain committed to meeting the needs of additional demographics who continue to struggle with this condition."
The endorsement of Adbry is grounded on outcomes from the Phase 3 ECZTRA 6 study. This trial assessed the treatment's effectiveness and security in treating 289 pediatric subjects, aged between 12 and 17 years, with moderate-to-severe atopic dermatitis who were eligible for systemic therapy. Out of them, 98 individuals were administered an initial 300 mg dose of Adbry, which was then reduced to 150 mg biweekly until the 16th week. The study successfully achieved its primary and significant secondary goals.
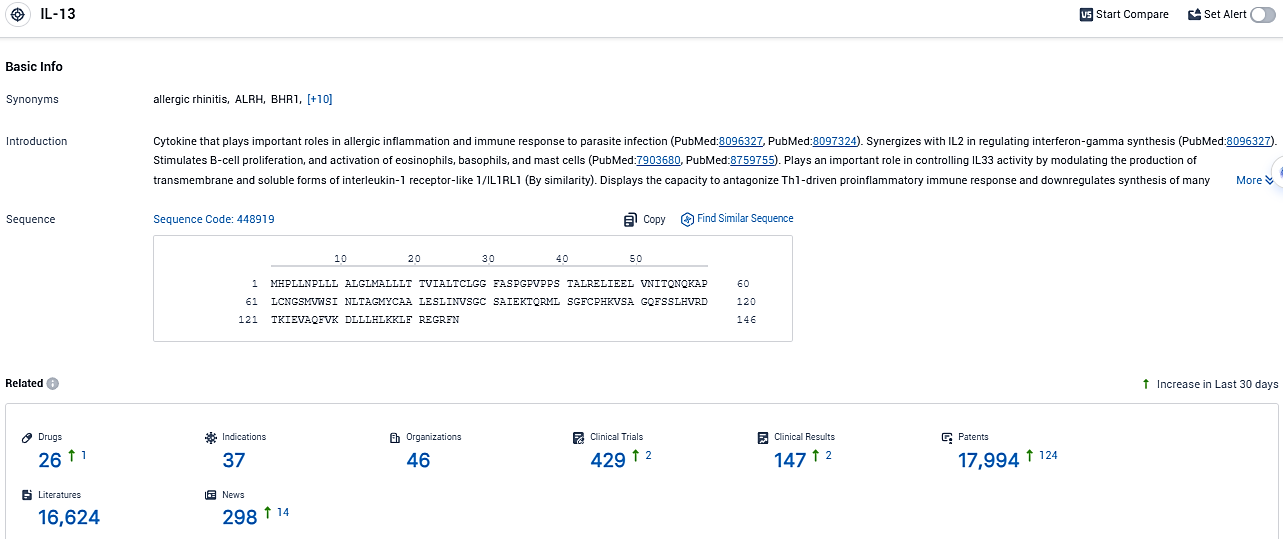
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 27, 2023, there are 26 investigational drugs for the IL-13 target, including 37 indications, 46 R&D institutions involved, with related clinical trials reaching 429, and as many as 17994 patents.
Adbry (tralokinumab-ldrm) is a high-affinity human monoclonal antibody developed to bind to and inhibit the interleukin (IL)-13 cytokine, which plays a role in the immune and inflammatory processes underlying atopic dermatitis signs and symptoms. Adbry, which is marketed outside of the U.S. under the tradename Adtralza(tralokinumab), is approved for the treatment of adults and pediatric patients with moderate-to-severe AD in the U.S., Canada, the European Union, the United Arab Emirates, Great Britain, and South Korea.