Lyell Immunopharma Reports Phase 1 Dose-dependent Efficacy of ROR1 CAR-T Therapy LYL797, Boosted by Anti-exhaustion Tech
Lyell Immunopharma, Inc. a clinical-stage company focused on T-cell reprogramming, has unveiled preliminary clinical and translational results from the Phase 1 trial of LYL797. This first-generation ROR1 CAR T-cell product candidate incorporates advanced anti-exhaustion technology and is part of their diverse pipeline of cell therapies targeting solid tumors.
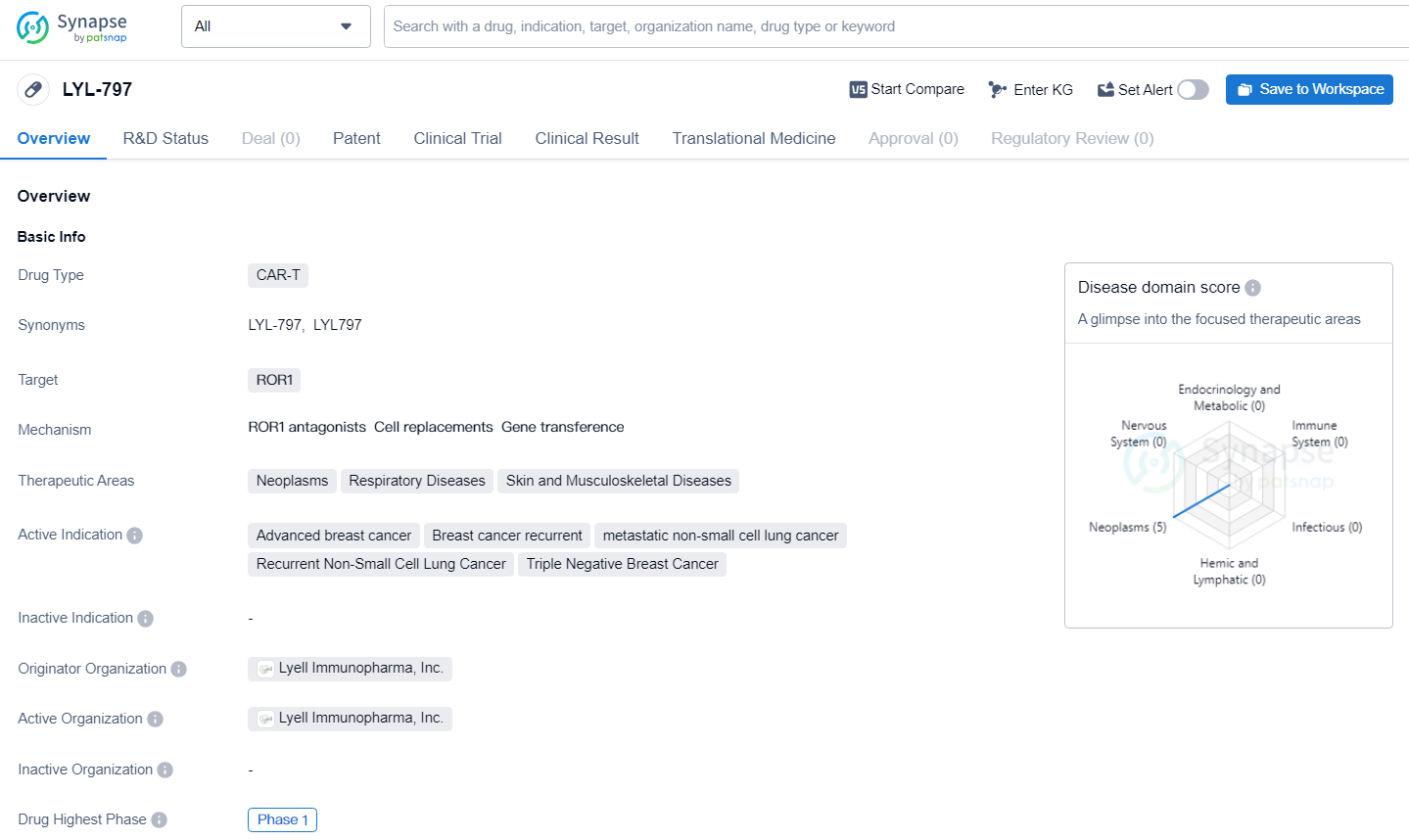
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The initial dataset predominantly includes patients with triple-negative breast cancer and showed dose-dependent antitumor clinical activity, as well as the ability of LYL797 CAR T cells to proliferate, infiltrate tumors, and eradicate cancer cells in patients with relapsed/refractory conditions. No cases of immune effector cell-associated neurotoxicity syndrome linked to LYL797 were reported. Pneumonitis was seen in patients with lung metastases, prompting a separate, more gradual dose escalation for these individuals. No dose-limiting toxicities were observed in patients without lung involvement. All patients now receive prophylactic steroids before LYL797 treatment.
“These initial clinical results are promising, showing that LYL797 ROR-1-targeted CAR T cells exhibited dose-dependent antitumor activity and hold the potential for even more substantial and lasting benefits as we proceed with dose escalation,” stated David R. Spigel, MD, Chief Scientific Officer at Sarah Cannon Research Institute, a medical oncologist, and a principal investigator in the LYL797 study. “Pneumonitis is a recognized complication of radiotherapy and multiple approved cancer therapies, including immune checkpoint inhibitors and various antibody-drug conjugates. We have adopted a protocol using steroids, the standard of care for pneumonitis treatment, which I believe will help us effectively monitor and manage these incidents.”
The LYL797 study includes a comprehensive translational program, revealing for the first time that CAR T cells enhanced with anti-exhaustion technology could expand, persist, and infiltrate solid tumors, sometimes showing evidence of cancer cell destruction. TIGIT, a marker for T cell exhaustion, was assessed in samples collected on Day 11 post-infusion, with only a small fraction of LYL797 CAR T cells being TIGIT-positive. RNAseq data indicated a significant proportion maintained the target phenotypes of stem-like and effector memory cells.
“We are heartened by the clinical responses and the clear dose-dependent antitumor activity observed with LYL797 in patients with advanced triple-negative breast cancer,” said Lynn Seely, MD, President and Chief Executive Officer of Lyell.
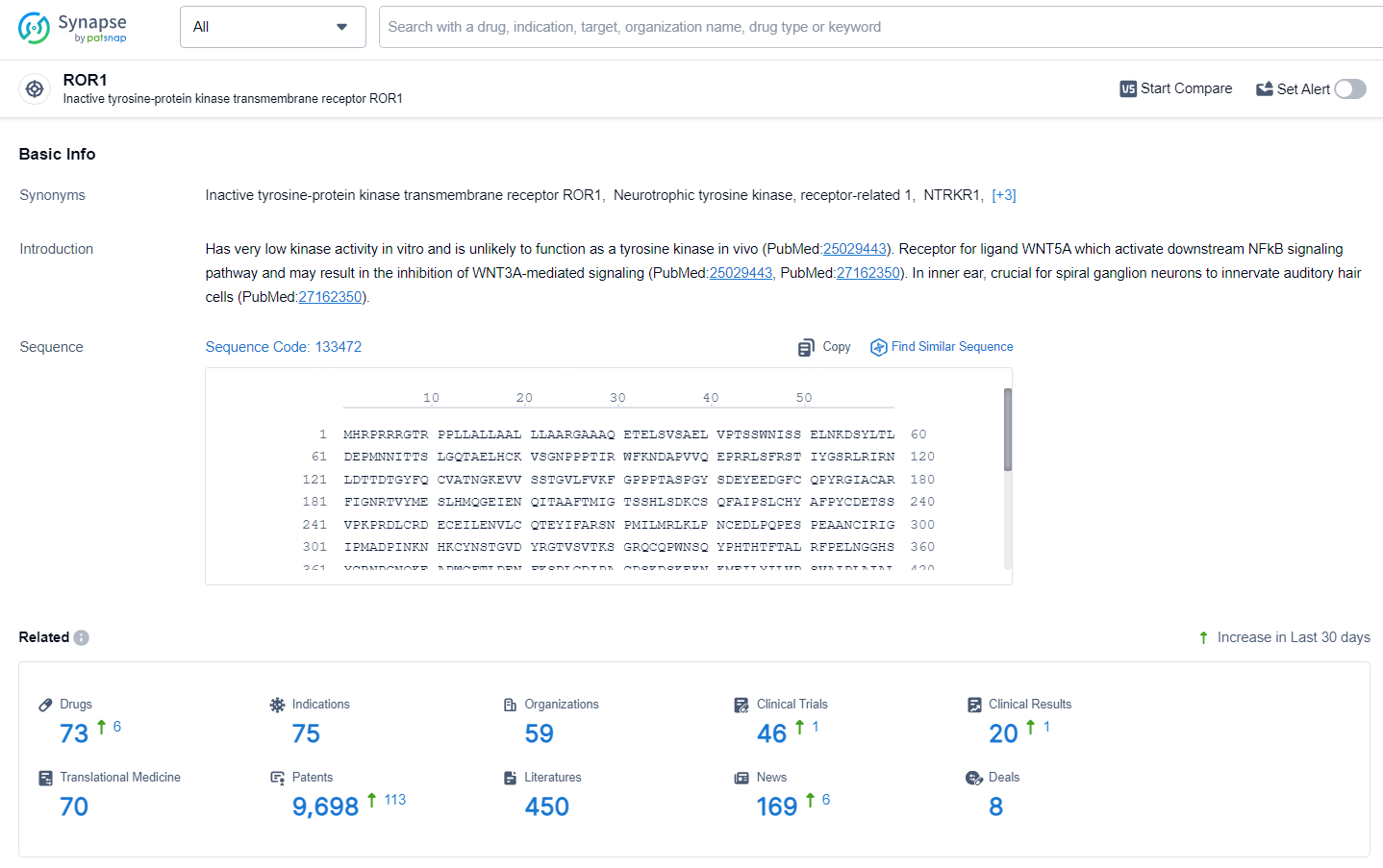
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 2, 2024, there are 73 investigational drugs for the ROR1 target, including 75 indications, 59 R&D institutions involved, with related clinical trials reaching 46, and as many as 9698 patents.
LYL-797 targets ROR1 and being developed for the treatment of various cancers and respiratory diseases. The drug has reached Phase 1 in its global development, indicating initial testing in humans to assess its safety and dosage. Further information on the drug's mechanism of action and clinical efficacy will be necessary to fully evaluate its potential impact in the pharmaceutical industry.