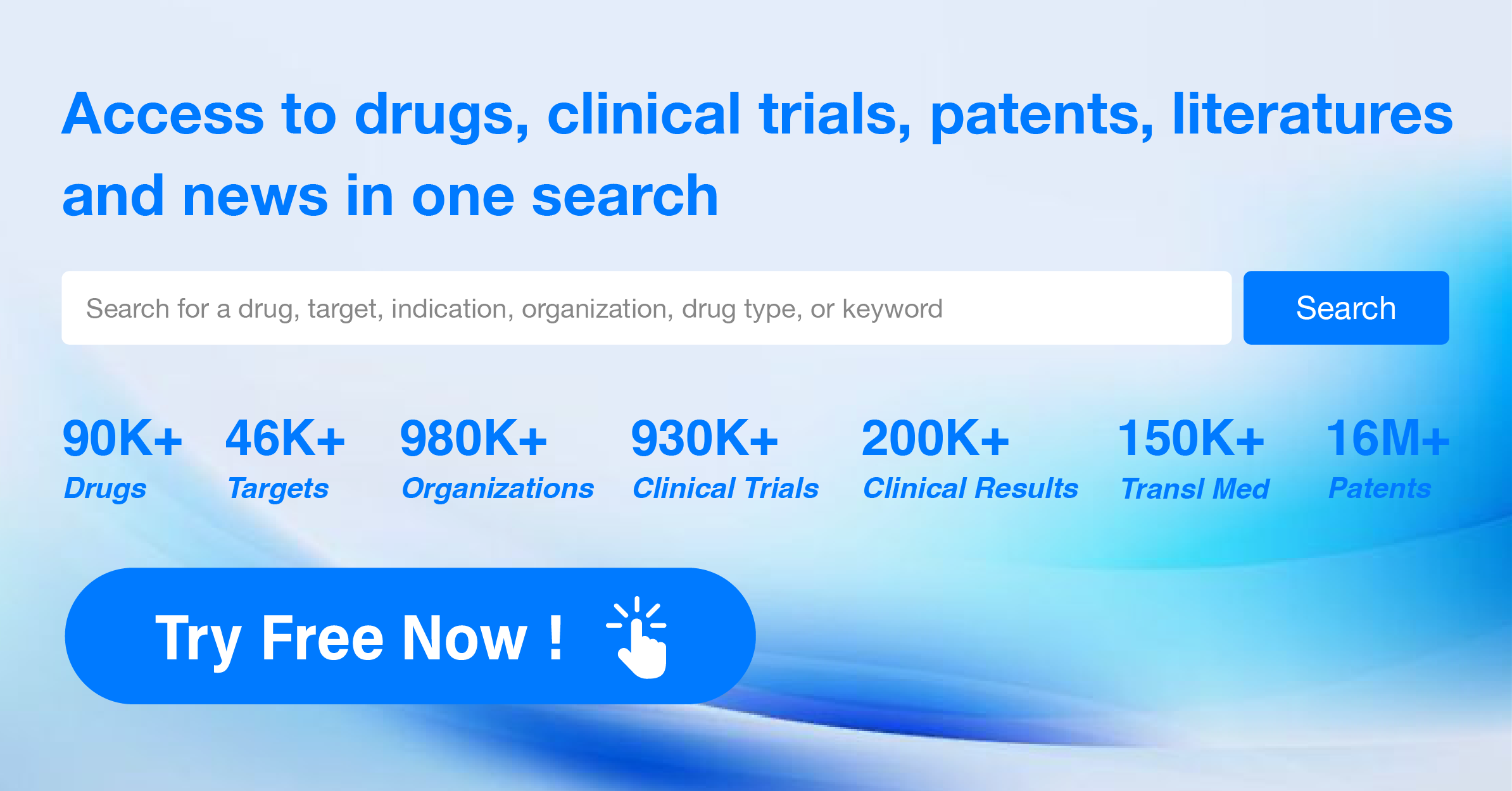
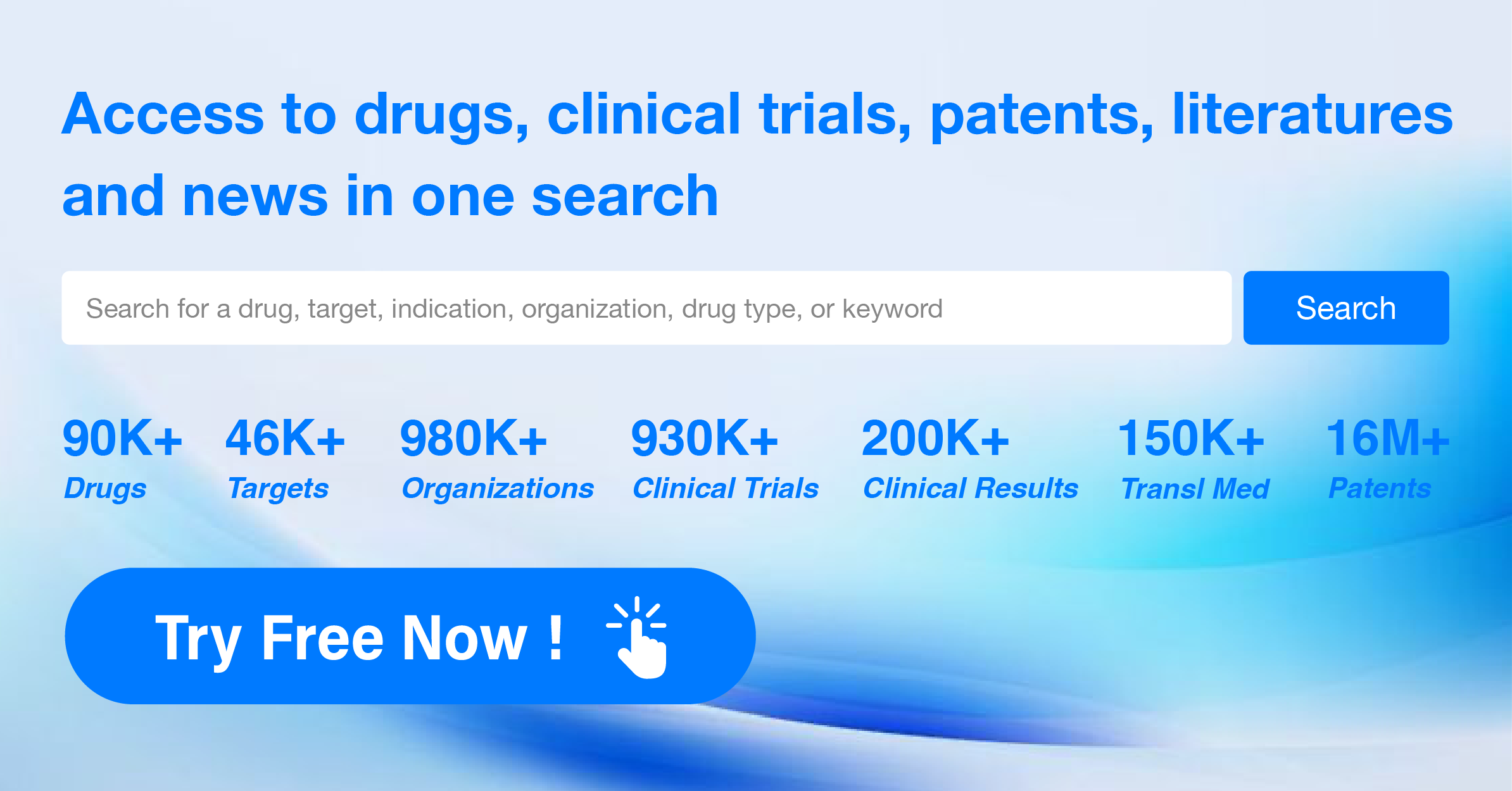
Global New Drug Research and Development Progress Weekly Report(6.24-6.30)
Global Pharmaceutical Research and Development Progress
1.Roche's Innovative Hematologic Cancer Drug Obinutuzumab Approved for Short-Duration Infusion Protocol in China
On June 24th, Roche announced that the NMPA has officially approved an update to the prescribing information for its hematologic cancer drug, Obinutuzumab injection, to include a 90-minute rapid administration Short Duration Infusion (SDI) protocol. It is understood that this protocol reduces the single infusion time of Obinutuzumab from the original 3-4 hours to just 90 minutes and can be initiated as early as the second treatment cycle of the patient, potentially becoming the preferred infusion protocol for patient treatment. The approval of the Obinutuzumab SDI protocol is based on data from the international multicenter Phase IV GAZELLe study. Data indicates that the SDI protocol has a good safety profile: there were no patients who experienced Grade ≥3 Infusion-Related Reactions (IRR) during the second-cycle SDI; only one patient experienced a Grade 3 IRR in subsequent cycles, with no incidences of Grade 4 or 5 IRR. In terms of duration, the median infusion time for the SDI protocol was 95-98 minutes, significantly reducing the administration time by more than half compared to the standard infusion protocol, which takes 205-269.5 minutes. Efficacy-wise, the SDI protocol achieved an Overall Response Rate (ORR) of 86.7%, consistent with the 88.5% reported in the Phase III GALLIUM study.
2.Novo Nordisk's semaglutide injection has been approved once again in China, indicated for long-term weight management
On June 25, Novo Nordisk announced that its once-weekly 2.4mg injection of semaglutide (trade name: Wegovy) has been approved in China for chronic weight management. Wegovy's weight loss indication was first approved by the U.S. FDA in 2021, for use in conjunction with diet and exercise for long-term weight management in adults with obesity or overweight who have at least one weight-related comorbidity. This also marked the first new drug approved by the FDA since 2014 for the management of general obesity or overweight. The approval of this product's weight reduction indication in China signifies that this breakthrough medication is poised to benefit a vast number of people with obesity and overweight in the country. According to a previous press release from Novo Nordisk, the FDA approval of Wegovy was based on the results of the STEP 3a phase clinical study program. In patients participating in various trials of the program without type 2 diabetes, those with obesity who consistently received Wegovy treatment for 68 weeks achieved an average weight reduction of 17% to 18%. Moreover, the product demonstrated favorable safety and tolerability throughout the program, with gastrointestinal events being the most commonly reported adverse effects.
3.Alnylam's Subcutaneous RNAi Therapy Amvuttra to Submit Regulatory Application Soon
On June 25, Alnylam Pharmaceuticals announced positive top-line results from its Phase III HELIOS-B clinical study. The analysis demonstrated that its subcutaneous RNAi therapy, Amvuttra (vutrisiran), as a monotherapy, reduced the risk of all-cause mortality or recurrent cardiovascular events by 33% in patients with ATTR amyloidosis with cardiomyopathy (ATTR-CM). Following these positive results, Alnylam plans to use a Priority Review Voucher (PRV) to submit a supplemental New Drug Application (sNDA) to the U.S. FDA. HELIOS-B is a global multicenter, randomized, double-blind, placebo-controlled Phase III study designed to evaluate the efficacy and safety of vutrisiran in reducing all-cause mortality and recurrent cardiovascular events in patients with ATTR-CM (primary endpoint). The study met its primary endpoint, with analysis during the double-blind period showing a reduction in the composite index of all-cause mortality and recurrent cardiovascular events by 28% and 33% for the overall patient group (n=654; HR: 0.718, p=0.0118) and the monotherapy-treated patients (i.e., patients not treated with the approved ATTR-CM therapy, tafamidis, at baseline, n=395; HR: 0.672, p=0.0162), respectively.
4.Johnson & Johnson Announces Approval of Teclistamab Injection in China for the Treatment of Multiple Myeloma
On June 25, Johnson & Johnson announced that the National Medical Products Administration (NMPA) of China has approved the marketing application for Teclistamab injection. Teclistamab is a ready-to-use, subcutaneous injectable therapy targeting both BCMA and CD3 as a bispecific antibody. The approved indication for Teclistamab is for monotherapy in adult patients with relapsed or refractory multiple myeloma (RRMM) who have previously been treated with at least three therapies. According to a Johnson & Johnson press release, clinical validation has shown that Teclistamab achieves a high response rate in patients with RRMM who have received at least three prior lines of therapy, with particularly better outcomes in Chinese patients, exhibiting an overall response rate (ORR) of 76.9%.
Teclistamab's safety and efficacy in the treatment of adult patients with RRMM were previously demonstrated in a clinical trial named MajesTEC-1. Following the positive results of this study, Teclistamab was consecutively approved by the European Union and the FDA in 2022 for the treatment of adult patients with RRMM. As detailed in an earlier press release by Johnson & Johnson, it is the first approved dual-specificity therapy for the treatment of multiple myeloma and the first approved bispecific antibody targeting BCMA, offering a “ready-to-use” treatment option for patients with refractory conditions.
5.Ionis Pharmaceuticals' new antisense oligonucleotide drug Olezarsen receives FDA priority review
On June 25, Ionis Pharmaceuticals announced that the FDA has accepted the New Drug Application (NDA) for Olezarsen for the treatment of familial chylomicronemia syndrome (FCS) and granted it priority review status, with a PDU LA date set for December 19, 2024. The NDA submission was primarily based on data from the Phase III Balance study. The Balance study is a global, multicenter, randomized, double-blind, placebo-controlled Phase III clinical trial that enrolled 66 adult patients with FCS, who received background therapies including statins, fibrates, and omega-3 fatty acids. In this study, patients were randomly assigned in a 1:1:1 ratio to receive subcutaneous injections of 50 mg, 80 mg of Olezarsen or a placebo every 4 weeks for 53 weeks. The primary endpoint was the percentage change from baseline in fasting triglyceride levels at 6 months compared to placebo. Results indicated that, in the 80 mg dose group, Olezarsen achieved the primary endpoint, with a statistically significant reduction in triglyceride levels adjusted for placebo from baseline at 6 months (44%, p<0.001); from 6 months to 12 months, triglyceride levels continued to decrease, with a reduction of 59% after placebo adjustment; apoC-III levels decreased by 74% and 81% from baseline at 6 months and 12 months, respectively, after placebo adjustment.
6.Roche Announces EU Approval of Subcutaneous Injection Formulation of CD20-Targeted Antibody Ocrevus
On June 26, Roche announced that the European Commission has approved the subcutaneous injection formulation of its CD20-targeted antibody, Ocrevus (ocrelizumab), for the treatment of relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). Previously released clinical results revealed that Ocrevus' subcutaneous injection formulation, requiring just two doses a year with each dose administered in only 10 minutes, nearly completely inhibited clinical relapses and brain lesions. According to the press release, Ocrevus is the first therapy approved for simultaneous treatment of both RMS and PPMS. This approval was largely based on pivotal data from the phase III clinical trial OCARINA II, which demonstrated that the serum levels of the subcutaneous injection formulation of Ocrevus were non-inferior to its intravenous infusion, and its safety and efficacy in patients with RMS and PPMS were comparable to the intravenous formulation. The subcutaneous formulation of Ocrevus was well-tolerated with no new safety issues identified. Long-term follow-up results published in April of this year revealed that subcutaneous Ocrevus (920 mg; n=236) nearly completely suppressed relapse activity during the treatment phase, with 97.2% of patients experiencing no relapses.
7.AbbVie's CD3/CD20 Bispecific Antibody Epcoritamab Approved in the US for Follicular Lymphoma
On June 26, AbbVie announced that the FDA has approved Epcoritamab (brand name: Epkinly) for the treatment of patients with relapsed or refractory follicular lymphoma (R/R FL) who have received at least two prior systemic therapies. Follicular lymphoma (FL) is a common type of B-cell non-Hodgkin's lymphoma (NHL), accounting for about 20%-30% of all NHL cases. The FDA approval was based primarily on the positive results from the Phase I/II EPCORE™NHL-1 study. This global, multicenter, open-label clinical trial enrolled 128 adult patients with CD20-positive (CD20+) R/R FL who had received at least two prior systemic therapies, one of which included a CD20 antibody. The overall response rate (ORR) assessed by an Independent Review Committee (IRC) was 82%, exceeding the pre-specified efficacy threshold. At a median follow-up of 14.8 months, the median duration of response (DOR) had not been reached (95% CI: 13.7, NR), with an estimated 68.4% of patients achieving a DOR of 12 months. Regarding safety, no new safety signals were observed. The most common treatment-emergent adverse events (TEAEs) were cytokine release syndrome (66.4%), with 1.6% of these events being grade >2 in severity.
8.Novo Nordisk's oral dual agonist amycretin has been approved for clinical trials in China
On June 26th, the official website of China's National Medical Products Administration’s Center for Drug Evaluation (CDE) announced that Novo Nordisk's Class 1 new drug, amycretin tablets, have been approved for clinical trials. The drug is intended to reduce the weight of adults who are overweight or obese. In a small phase 1 clinical trial previously announced by Novo Nordisk, patients treated with amycretin showed a significant weight reduction of 13.1% after 12 weeks (compared to 1.1% in the placebo group), demonstrating rapid weight loss effects and good potential for clinical application. According to Novo Nordisk’s website, amycretin is a long-acting dual agonist of GLP-1R and insulin receptor. It is currently being developed in two formulations: a subcutaneous injection (once weekly) and an oral tablet (once daily), with the intended indication for obesity. In March of this year, Novo Nordick released the latest clinical trial results for oral amycretin. This was a small phase 1 trial involving 16 patients, where those treated experienced a significant weight loss of 13.1% over 12 weeks, compared to a marginal reduction of 1.1% in the placebo group. Additionally, amycretin demonstrated good pharmacokinetic properties, as well as safety, tolerability, and adverse reactions similar to those of other GLP-1 therapies under development.
9.CSPC Pharmaceutical's PD-1 Antibody Enlonstobart Injection Approved for Market
On June 28th, the marketing authorization application for the PD-1 antibody Enlonstobart injection, developed by CSPC Megalith Biopharmaceutical, a subsidiary of CSPC Pharmaceutical Group, was approved by the Drug Administration Authority. The approved indication for this product is for the treatment of patients with PD-L1 positive recurrent or metastatic cervical cancer who have failed at least one line of platinum-containing chemotherapy. Safety and efficacy were further confirmed by the phase II study (NCT04886700) presented at the ASCO 2024 conference. The phase II study enrolled 107 patients with PD-L1 positive (CPS≥1) cervical cancer who had either progressed during or after first-line platinum-based treatment, or were intolerant to it. Patients were administered 240 mg of Enlonstobart every two weeks for a treatment period up to 24 months or until disease progression, intolerable toxicity, or other study termination criteria were met. Results showed that, after a median follow-up of 13.96 months, the objective response rate (ORR) and disease control rate (DCR) were 29.0% (95% CI, 20.6-38.5) and 54.2% (95% CI, 44.3-63.9) respectively, including two complete responses and 29 partial responses. The median duration of response (mDoR) was 16.6 months (95% CI, 10.8-NA), with a median progression-free survival (mPFS) of 3.1 months (95% CI, 2.2-6.9).
10.Incyte Corporation's axatilimab injection approved for clinical trials in China
On June 27th, the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration published an announcement that Incyte Corporation's new drug application for axatilimab injection, a first-class drug, was approved for clinical trials. The drug is intended for the treatment of relapsed or refractory active chronic graft-versus-host disease (cGVHD) in patients who have previously undergone systemic therapy. Public information reveals that axatilimab is an antibody targeting the Colony-Stimulating Factor-1 Receptor (CSF-1R). The product's marketing application for treating cGVHD has been granted priority review by the U.S. Food and Drug Administration (FDA). In February of this year, Incyte announced that the FDA had accepted the Biologics License Application (BLA) for axatilimab and granted it priority review status for the treatment of cGVHD after at least two prior systemic therapies had failed, with a Prescription Drug User Fee Act (PDUFA) target action date set for August 28, 2024. The BLA submission was supported by positive data from the pivotal phase 2 AGAVE-201 trial. The AGAVE-201 study assessed the efficacy and safety of axatilimab in adults and children with refractory cGVHD who had previously received at least two systemic treatments. The study achieved its primary endpoint across all cohorts: patients receiving a dose of 0.3 mg/kg of axatilimab every two weeks achieved a peak overall response rate of 74% within the first six months of treatment.
For more information on the progress of drug development, please follow the Synapse database.
Dynamics of Global Pharmaceutical Trade Cooperation
1.Walvax Biotechnology Terminates Collaboration on Two mRNA Vaccines with Abogen Biosciences
On June 25, Walvax Biotechnology and Abogen Biosciences terminated their technical development collaboration on both the novel coronavirus mRNA vaccine and the herpes zoster mRNA vaccine, and signed a Termination Agreement. Following the termination of this collaboration, the parties will settle costs related to prior R&D and clinical trials. The board has authorized the company's chairman and executive management to handle matters related to the termination, including but not limited to signing the termination agreement, settlement agreements, related ancillary documents, and settling project costs. The cooperation between Walvax Biotechnology and Abogen Biosciences began in May 2020, when both parties signed a Technical Development Cooperation Agreement aimed at jointly developing a novel coronavirus mRNA vaccine. Under the agreement, Abogen Biosciences was responsible for the preclinical research of the vaccine, including mRNA vaccine molecular design, chemical modification, and formulation process development, while Walvax Biotechnology was responsible for the vaccine's QA, QC, IND, clinical studies, as well as NDA and commercial production.
2.Eli Lilly and Company Announces Collaboration with OpenAI to Develop Innovative Antibiotics
On June 26th, Eli Lilly and Company announced a collaboration with OpenAI to use OpenAI's generative AI for the discovery of new antibiotics to treat drug-resistant pathogens. Generative artificial intelligence opens new opportunities for accelerating the discovery of novel antimicrobial drugs and developing tailored technologies to combat antibiotic-resistant pathogens. This collaboration highlights our commitment to addressing the significant health challenges faced by people around the world. Previously, the portfolio has committed $100 million to the AMR Action Fund, aiming to provide patients with two to four new antibiotics by 2030 as the next line of defense against multi-drug resistant pathogens.
3.Novo Nordisk Acquires Gene Editing Therapies and Platform
On June 26, 2seventy bio announced that it has reached an Asset Purchase Agreement (APA) with Novo Nordisk. According to the terms of the APA, Novo Nordisk gains interests in the hemophilia A project and 2seventy bio's in vivo gene editing technology platform, which is primarily used to develop autologous or allogeneic cell therapies for autoimmune diseases. Following the agreement, the 2seventy bio team involved in developing this technology platform will join Novo Nordisk and continue their development efforts. Under the terms of the contract, 2seventy bio could receive up to $40 million in payments. After the completion of the asset divestiture, 2seventy bio will focus on the commercialization and ongoing development of Abecma (idecabtagene vicleucel). Initially, the collaboration agreement reached between 2seventy bio and Novo Nordisk in 2019 was to develop gene editing therapies for hemophilia A. Abecma, a BCMA-targeted CAR-T cell therapy, was approved in the United States in March 2021 for the treatment of patients with relapsed or refractory multiple myeloma (R/R MM) who have received at least four prior therapies including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. In April this year, its application was expanded to include third-line treatments.
4.AbbVie Announces $250 Million Acquisition Deal! Secures Potential "First-in-Class" Therapy
On June 28, AbbVie announced its acquisition of Celsius Therapeutics for $250 million. Celsius Therapeutics is a clinical-stage biotechnology company focused on developing novel drugs for the treatment of inflammatory diseases. The company's lead candidate, CEL383, is a potential "first-in-class" TREM1-targeting antibody intended for the treatment of inflammatory bowel disease (IBD). Celsius Therapeutics employs a research and development model that involves single-cell RNA sequencing of patient tissue, followed by the application of machine learning and artificial intelligence to analyze the vast amount of generated data to identify factors driving disease. Many diseases may originate from interactions or joint dysfunction among various types of cells, and traditional gene sequencing, which averages readings over multiple cells, might fail to detect these disease-causing variations. By using single-cell RNA sequencing, Celsius can achieve very high-resolution insights into gene expression and cellular behavior changes. This information, combined with machine learning, could offer insights into entirely new therapeutic targets.