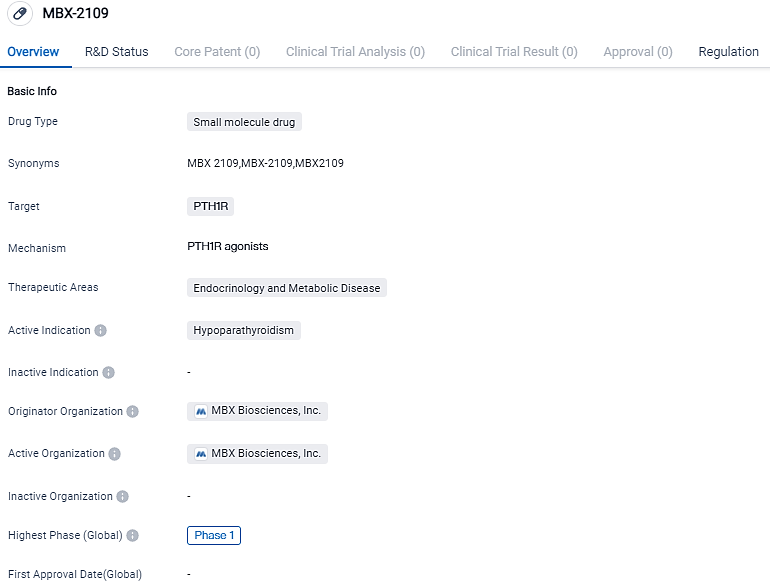
MBX Biosciences declares encouraging data from a Phase 1 Multiple Ascending Dose study for MBX 2109
MBX Biosciences, Inc., a biopharmaceutical firm engaged in clinical trials, released favorable outcomes from the Phase 1 trial's multiple ascending dose section for MBX 2109. This long-acting parathyroid hormone (PTH)) peptide prodrug developed by the company is a type of Precision Endocrine Peptide™ (PEP™) therapies aimed at handling a variety of endocrine disorders, specifically hypoparathyroidism.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We're encouraged by the promising data from our Phase 1 study, reinforcing our confidence in the potential advantages of the PEP technology platform," stated MBX Biosciences' CEO and president, Kent Hawryluk. "MBX 2109, our principal product candidate, and a potential market leader in PTH replacement therapy, is geared to progress to the next phase of its development. We are enthusiastically preparing for our Phase 1 conclusion conversation with the FDA later this year."
A total of 76 adults participated in a randomized, double-blind, placebo-controlled Phase 1 clinical trial meant to measure the safety, tolerability, PK and PD of individual and multiple escalating doses of MBX 2109 in healthy adult volunteers. Single and multiple increasing doses of MBX 2109 proved largely acceptable in terms of tolerance with primary and secondary endpoints being safety, tolerability, respective PK and PD parameters.
Hypoparathyroidism, which affects approximately 200,000 people globally, is a rare hormonal disease resulted from an inadequate supply of parathyroid hormone, leading to reduced calcium and amplified phosphorus levels in the bloodstream. Typical triggers of HP are parathyroid gland damage or removal during thyroid surgery. The condition is typified by a wide spectrum of symptoms and comorbidities and the current primary treatment aim is to consistently manage blood calcium levels toward the lower end of the normal spectrum, hence alleviating hypocalcemia symptoms.
The recommended treatment nowadays encompasses high-dose calcium supplementations and active vitamin D, potentially contributing to complications with the kidneys and not targeting the basal pathophysiology induced by a parathyroid hormone deficiency.
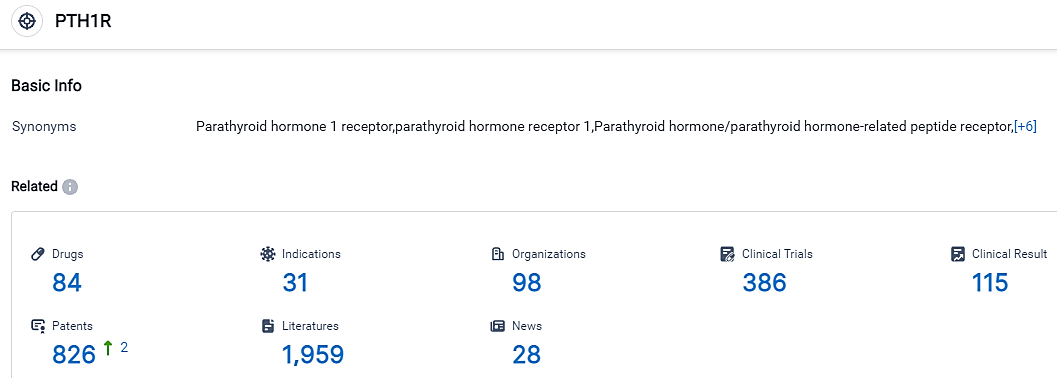
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 14, 2023, there are 84 investigational drugs for the PTH1R target, including 31 applicable indications,98 R&D institutions involved, with related clinical trials reaching 386,and as many as 826 patents.
MBX 2109 is an investigational long-acting parathyroid hormone peptide prodrug in development as a PTH replacement therapy for hypoparathyroidism. In July 2022, MBX 2109 received Orphan Drug designation from the U.S. Food and Drug Administration for the treatment of hypoparathyroidism. MBX aims to simplify and improve an individual’s disease management, while relieving both the symptoms of the disorder and long-term complications.