First Non-Hormonal Targeted Therapy: A New Breakthrough in Menopausal Hot Flashes
On May 12, 2023, a non-hormonal targeted therapy called Fezolinetant (trade name VEOZAH) was approved and launched in the United States, making it the world's first non-hormonal targeted drug for the treatment of moderate to severe vasomotor symptoms (VMS) caused by menopause. In this article, we will provide a detailed analysis of Fezolinetant's mechanism of action, safety advantages, clinical efficacy, research and development progress, and competitive landscape, aiming to help colleagues gain a better understanding of this product.
Unlike hormone-based medications, Fezolinetant primarily works by antagonizing the NK3 receptor. The NK3 receptor, also known as the neurokinin 3 receptor, belongs to the neuropeptide Y (NPY) receptor family and is mainly distributed in the central nervous system, particularly in the cerebral cortex, hypothalamus, and hippocampus.
The NK3 receptor regulates various neural and behavioral activities such as appetite, sleep, and attention by binding to neuropeptide Y. In the hypothalamic region, the activation of NK3 receptors has a positive effect on the temperature-regulating center and counteracts the inhibitory effects of estrogen feedback. During menopause, the disruption of this balance due to decreased estrogen levels leads to the occurrence of VMS.
Fezolinetant has a high affinity for the NK3 receptor (Ki value of 19.9-22.1 nmol/L), which is more than 450 times higher than its affinity for NK1 or NK2 receptors. By antagonizing the NK3 receptor, Fezolinetant blocks the binding of neurokinin B (NKB) to kisspeptin/neurokinin B/dynorphin (KNDy) neurons, modulating the activity of the temperature-regulating center's neurons and ultimately restoring balance and relieving VMS. Compared to direct supplementation of estrogen, this treatment mechanism results in fewer side effects.
If a woman has gone through 12 consecutive months without menstruation, she has entered menopause, which is a normal natural change that usually occurs between the ages of 45 and 55. During menopause, the production of estrogen and progesterone in a woman's body gradually decreases, and approximately 80% of menopausal women will experience vasomotor symptoms, including brief episodes of sweating, facial flushing, and cold sensations.
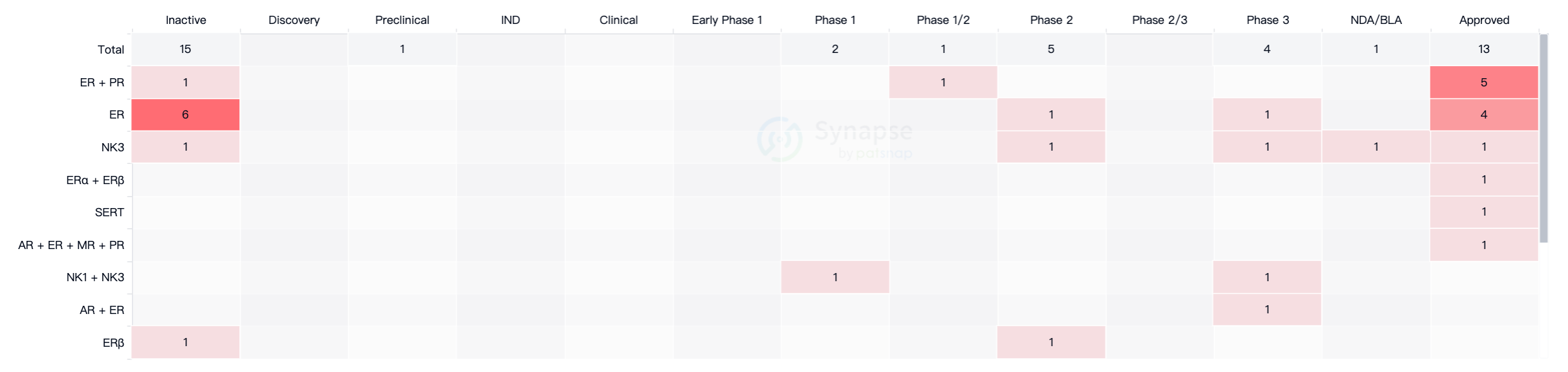
For menopausal women with significant symptoms, the main treatment methods currently include hormone replacement therapy, which involves the use of estrogen, progesterone, or combined estrogen-progesterone therapy. According to data from the Intelligence Blossom New Drug Information Database, as of May 23, 2023, there are a total of 33 drugs worldwide for the treatment of vasomotor symptoms, of which 13 have been approved for market, but apart from Fezolinetant, all others are hormone-based medications (Figure 1).
However, long-term use of hormone-based medications may lead to a range of negative effects, such as decreased appetite, changes in blood lipid levels, edema, hormone-related obesity, and in severe cases, it may also lead to endometrial hyperplasia, hypertension, osteoporosis, diabetes, and liver damage.
In addition, some women who have a history of vaginal bleeding, stroke, heart attack, thrombosis, or liver disease are not suitable for hormone-based treatments.
Clinical studies have shown that Fezolinetant rarely causes severe adverse events, and the most common side effects include abdominal pain, diarrhea, insomnia, back pain, facial flushing, and elevated liver enzymes.
Compared to hormone-based medications, Fezolinetant has fewer and milder side effects and is suitable for a wider range of individuals, offering significant therapeutic advantages. The approval of Fezolinetant provides a better choice for the treatment of vasomotor symptoms.
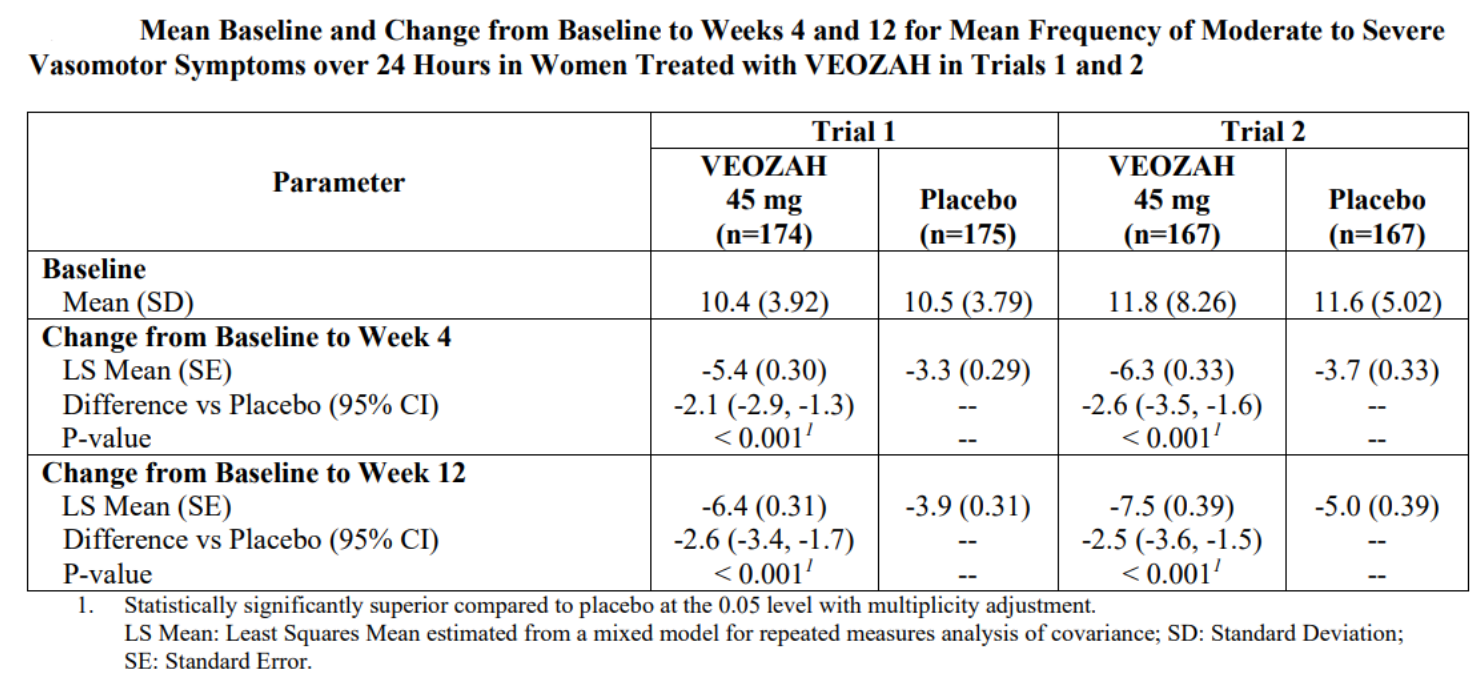
Based on the results of two randomized, double-blind, placebo-controlled Phase 3 clinical trials [2], the FDA approved the marketing application for Fezolinetant. In these two trials, subjects (with an average age of 54) were randomly assigned to receive either placebo or Fezolinetant for the first 12 weeks. After 12 weeks, subjects in the Fezolinetant group continued their previous treatment, while subjects in the placebo group were randomly assigned to receive either 30 mg or 45 mg of Fezolinetant and underwent a 40-week treatment period. The primary efficacy endpoints were the frequency and severity of vasomotor symptoms (VMS) at Week 4 and Week 12.
The final results showed that both doses of Fezolinetant, compared to placebo, significantly reduced the frequency/severity of VMS at Week 4 and Week 12 in a statistically significant manner (Figure 2). The frequency and severity of VMS showed improvement as early as Week 1 of treatment and this improvement was sustained for 52 weeks.
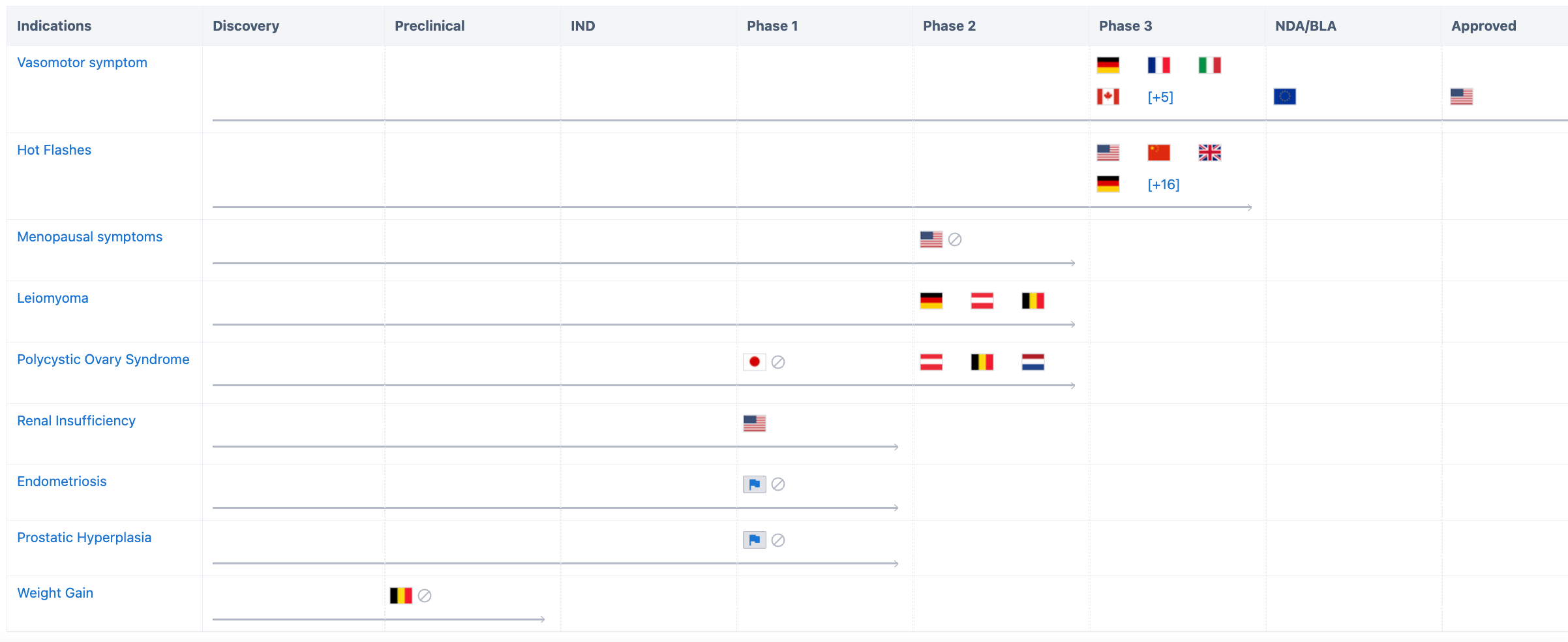
In addition to being approved for the treatment of vasomotor symptoms (VMS), Fezolinetant is also being positioned for multiple indications in various countries/regions. Due to limited development of similar drugs, Fezolinetant holds a distinct competitive advantage in the NK3 pathway.
Fezolinetant's extensive positioning in different indications suggests broad future applications.
Firstly, in terms of indications, according to Synapse Database, Fezolinetant has already covered multiple indications such as vascular diseases, hot flashes, uterine fibroids, polycystic ovary syndrome, and renal diseases.
For the development of vasomotor symptoms, in addition to the approval obtained in the United States, Fezolinetant has submitted a marketing application in the European Union and has entered Phase 3 clinical trials in the United Kingdom, Canada, and Spain. In China, Phase 3 clinical trials are underway for indications related to vascular diseases and hot flashes.
Fezolinetant has relatively few similar products.
According to data from Synapse, there are currently 25 drugs targeting NK3 receptors worldwide, with most of them either terminated or not progressing. The drugs currently under active development include SCH-206272, which targets NK3, NK1, and NK2 receptors and is in the preclinical stage.
However, its indications are asthma, cough, and chronic obstructive pulmonary disease, and it does not compete directly with Fezolinetant. SJX-653 is in Phase 2 clinical trials and is developed solely for the indication of hot flashes. Ozenanetant is in Phase 2 clinical trials and is being studied for indications such as prostate cancer, vasomotor symptoms, and post-traumatic stress disorder. The main competitor is Elinzanetant, developed by Bayer, which is currently in Phase 3 clinical trials and focuses on indications related to menopausal syndrome.
There is some overlap in indications between Elinzanetant and Fezolinetant, but Elinzanetant has limited market presence in terms of countries/regions. As the world's first NK3 receptor antagonist to receive marketing approval, Fezolinetant already possesses a competitive advantage and is expected to maintain this competitiveness in the coming years, or even decades.
In summary, Fezolinetant not only significantly reduces the frequency and severity of VMS but also has fewer and milder side effects compared to hormone-based medications. It has a broader range of applications and demonstrates clear treatment advantages.
Due to the relatively limited development of similar drugs, Fezolinetant has a wider market presence in terms of countries and indications, and its research is more advanced. With the progress of various trials, Fezolinetant is poised to provide a treatment option for a broader population.




