Merck and EyeBio Initiate Phase 2b/3 Trial for Restoret™ in Diabetic Macular Edema Treatment
Merck (NYSE: MRK), recognized as MSD beyond the borders of the U.S. and Canada, along with EyeBio, a fully owned subsidiary of Merck & Co., Inc., headquartered in Rahway, New Jersey, USA, has announced the commencement of the Phase 2b/3 BRUNELLO study. This trial is examining the efficacy of Restoret™ (MK-3000, previously known as EYE103) for addressing diabetic macular edema (DME).
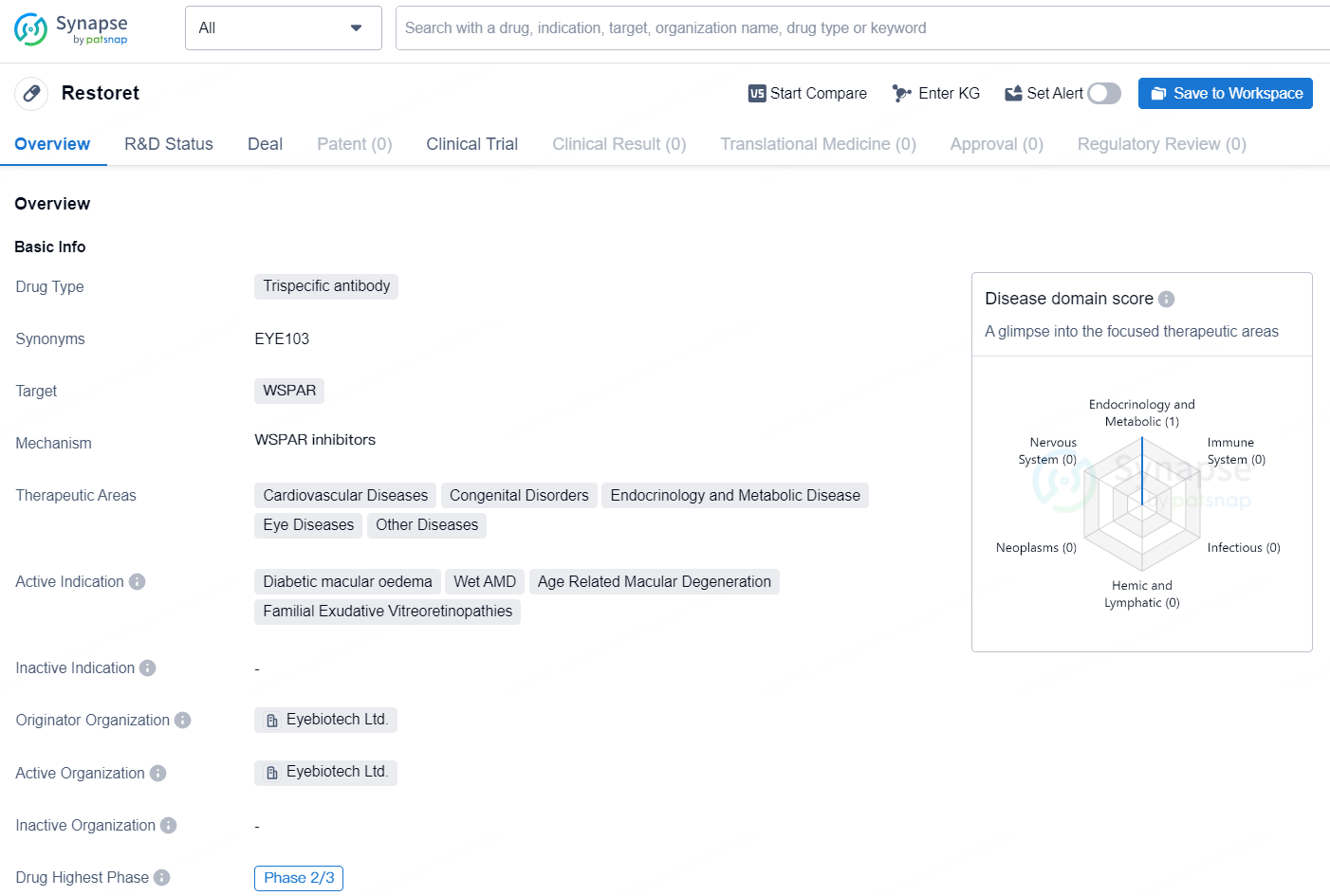
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MK-3000 is an investigative, potentially novel tetravalent, tri-specific antibody functioning as an agonist of the Wingless-related integration site (Wnt) signaling pathway. The commencement of the BRUNELLO trial is grounded in results from the open-label, Phase 1/2 AMARONE study of MK-3000 in subjects with diabetic macular edema (DME) and neovascular age-related macular degeneration (NVAMD).
“The data from the Phase 1/2 AMARONE study highlighted the potential impact of MK-3000 on patients with retinal disorders,” stated Dr. David Guyer, the founder, CEO, and president of EyeBio. “Launching the BRUNELLO trial is a crucial step as we collaborate with our new partners at Merck, united by the goal to offer new, urgently needed treatments for individuals with diabetic macular edema.”
BRUNELLO is a randomized and double-masked Phase 2b/3 trial (NCT06571045) that will investigate the efficacy and safety of two dosage levels of intravitreal (IVT) Restoret (MK-3000) in comparison to the active control, ranibizumab, in patients with DME. Eligible participants will be randomized in a 1:1:1 ratio to receive either low or high doses of MK-3000 or ranibizumab every four weeks during the first year. In the second year, treatment frequency will be adjusted based on a personalized treatment interval (PTI) algorithm. The primary dual endpoints are safety and the mean change in best-corrected visual acuity (BCVA) from baseline to week 52 in the study eye of the participants, measured using standardized Early Treatment of Diabetic Retinopathy Study (ETDRS) vision.
Restoret (MK-3000, previously EYE103) is an investigative, potentially novel tetravalent, tri-specific Wnt antibody created to meet unmet medical needs in patients with retinal diseases such as diabetic macular edema (DME) and neovascular age-related macular degeneration (NVAMD). MK-3000 is delivered as an intravitreal injection with the aim of eliminating vascular leakage in retinal conditions by agonizing the Wnt pathway, ultimately striving to restore and maintain the blood-retinal barrier. Preclinical studies suggest that Wnt pathway agonism in the retina may decrease vascular leakage.
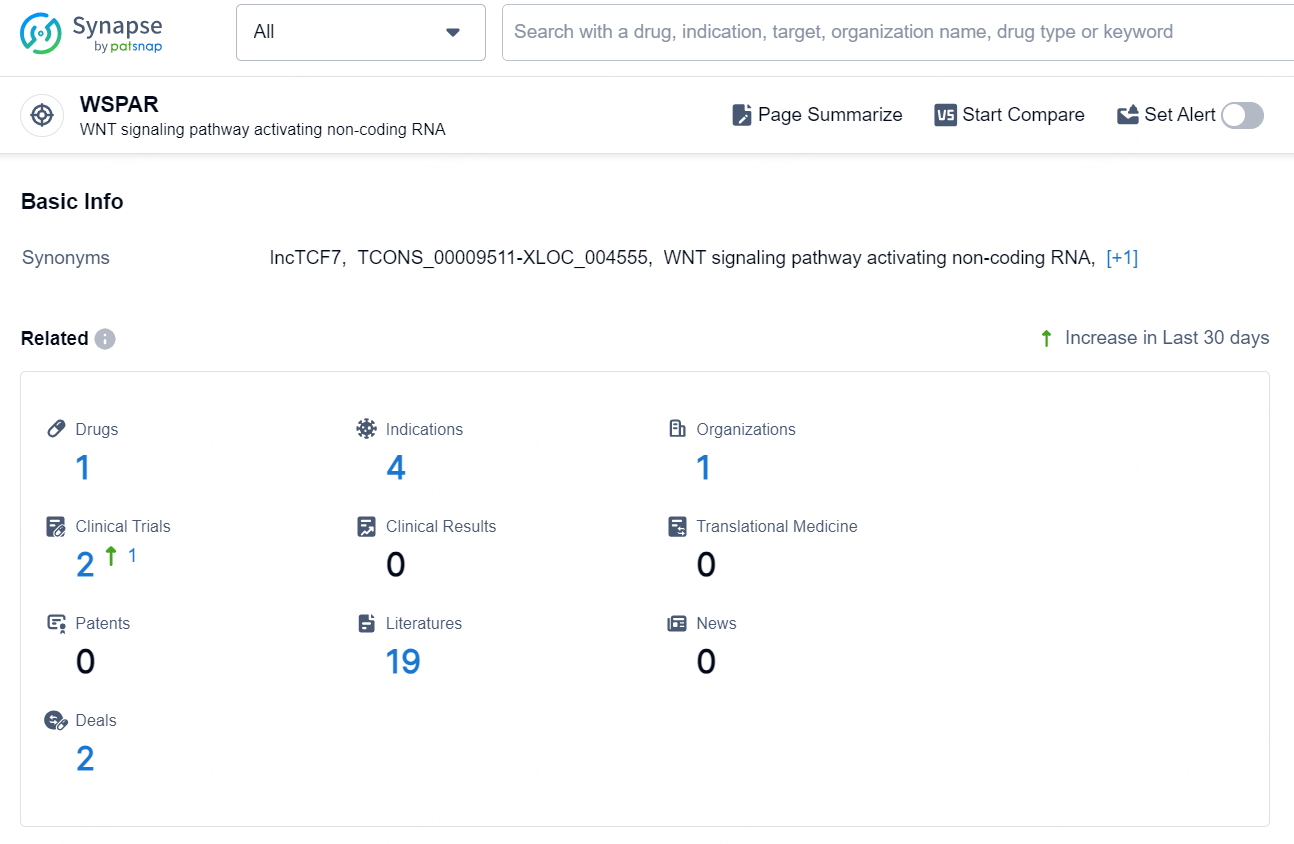
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 1 investigational drug for the WSPAR targets, including 4 indication, 1 R&D institution involved, with related clinical trials reaching 2.
Restoret is a trispecific antibody drug developed by Eyebiotech Ltd. The drug targets WSPAR and is being developed for the treatment of various therapeutic areas, including Cardiovascular Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, Eye Diseases, and Other Diseases. Restoret is currently indicated for the treatment of Diabetic macular oedema, Wet AMD (Age-related macular degeneration), and Familial Exudative Vitreoretinopathies. These conditions are all related to eye diseases and represent the main focus of the drug's development.