Vasa Therapeutics Initiates Phase 1 Trial of VS-041 for Heart Failure Treatment
Vasa Therapeutics ("Vasa"), a clinical-stage biopharmaceutical company focused on innovative treatments for cardiovascular and metabolic aging, announced that the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) has granted clinical trial authorization (CTA) to Vasa. This authorization allows Vasa to begin a Phase 1 first-in-human clinical trial aimed at assessing the safety, tolerability, and pharmacokinetics of its experimental drug VS-041 in healthy adult volunteers. VS-041 is an oral medication with a unique mechanism of action, potentially for treating heart failure with preserved ejection fraction (HFpEF).
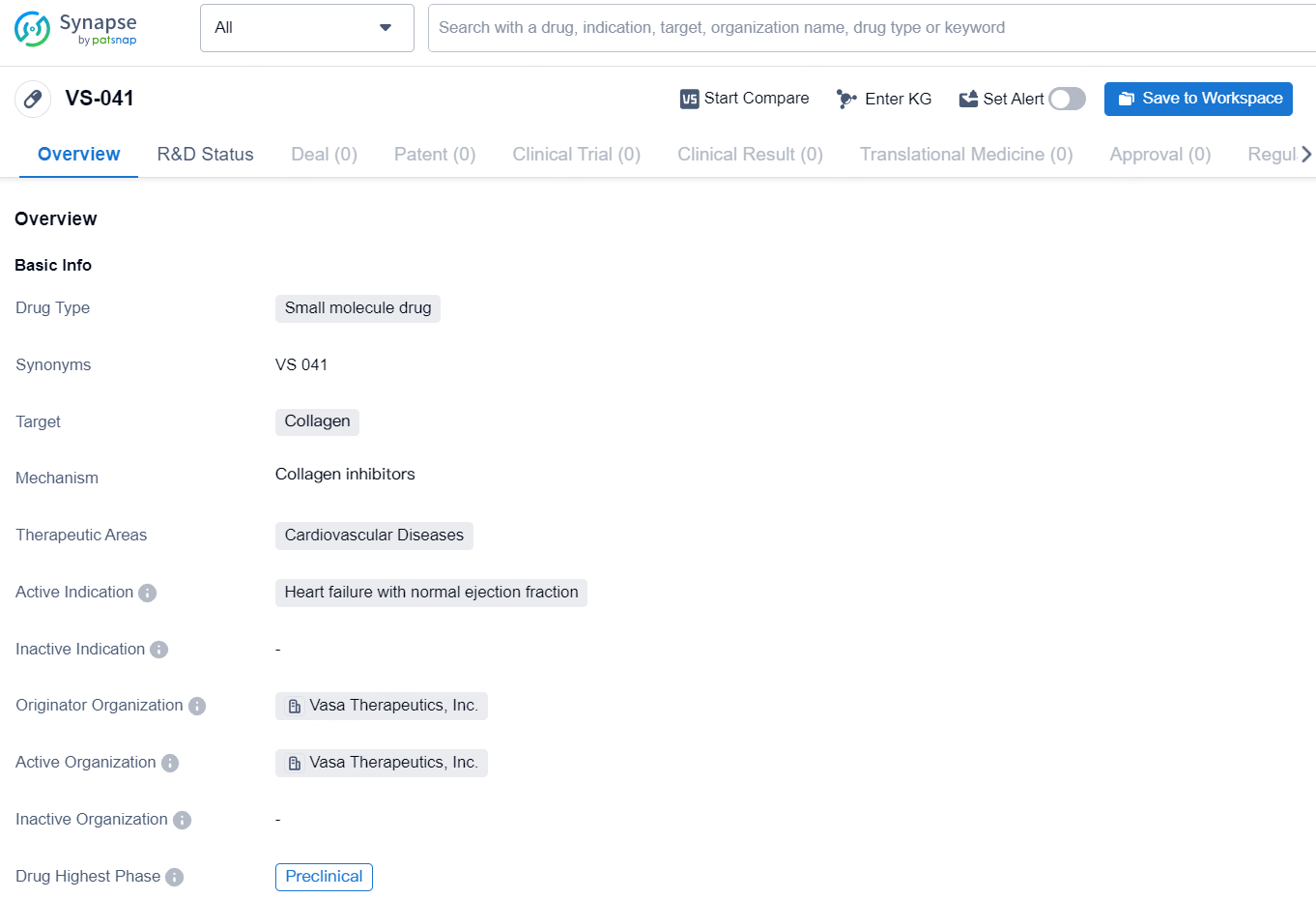
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The MHRA's approval represents a significant advancement in our goal to develop VS-041 as a disease-modifying therapy for HFpEF," stated Artur Plonowski, MD, PhD, CEO of Vasa Therapeutics. "Presently, no HFpEF treatments are available that enhance the structural characteristics of the myocardium or offer lasting clinical benefits. By inhibiting endotrophin release, a biomarker and mediator of fibrotic and inflammatory responses, VS-041 could become the first personalized treatment strategy for HFpEF, particularly for patients with elevated endotrophin levels and a high risk of poor outcomes."
The start of the Phase 1 trial for VS-041 coincides with the extension of the Company's seed funding, increasing the total to $11 million. The funding was spearheaded by Orphinic Scientific SA with contributions from i&i Biotech Fund I SCSp. The funds will be utilized to support the clinical development of VS-041 and further Vasa’s preclinical initiatives.
VS-041 is an orally administered compound identified and developed by Vasa for the prospective treatment of HFpEF. In preclinical HFpEF models, VS-041 significantly reduces cardiac fibrosis and improves diastolic heart function. The compound also suppresses the secretion of endotrophin from primary human cardiac fibroblasts. In GLP toxicology studies, VS-041 showcased an excellent safety and tolerability profile. The preclinical development of VS-041 received co-funding from the European Regional Development Fund and the Polish National Centre for Research and Development (POIR.01.01.01-00-1210/19-01).
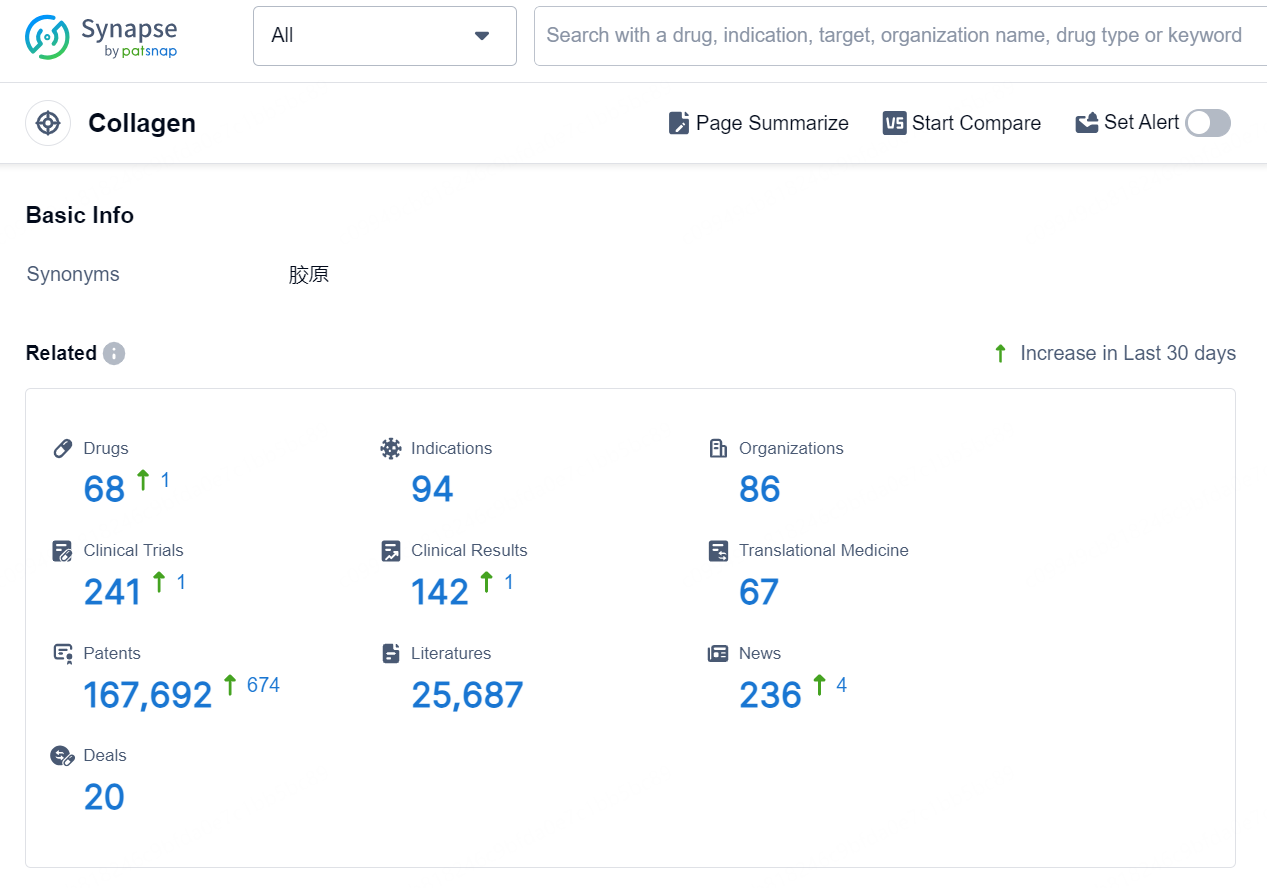
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 68 investigational drugs for the collagen targets, including 94 indications, 86 R&D institutions involved, with related clinical trials reaching 241, and as many as 167692 patents.
The drug VS-041 is a small molecule drug developed by Vasa Therapeutics, Inc. It targets collagen and is intended for the treatment of cardiovascular diseases, with a specific focus on heart failure with normal ejection fraction. As of the latest available information, the drug is in the preclinical phase, indicating that it is still undergoing laboratory and animal testing to assess its safety and efficacy.