Fate Therapeutics Debuts FT522 CAR NK Cell at 2024 ACR
Fate Therapeutics, Inc. (NASDAQ: FATE), a biopharmaceutical firm in the clinical development stage focused on delivering first-in-class therapies based on induced pluripotent stem cells (iPSCs) for the treatment of cancer and autoimmune diseases, has announced the preliminary clinical and translational findings from its Phase 1 trial of FT522 in patients with relapsed/refractory B-cell lymphoma. This was shared at the American College of Rheumatology (ACR) Convergence taking place in Washington, D.C.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
FT522 is the commercial off-the-shelf product candidate of the Company, designed as a CD19-targeted chimeric antigen receptor (CAR) natural killer (NK) cell therapy. This candidate features several innovative synthetic mechanisms that regulate cell functionality, specifically aimed at targeting and eliminating harmful immune cells. It is the Company’s inaugural candidate utilizing its alloimmune defense receptor (ADR) technology, allowing for effective treatments in patients without the need for intense conditioning chemotherapy. The Company has also commenced a Phase 1 trial of FT522, which will encompass a range of B cell-mediated autoimmune disorders, serving as an adjunct to standard induction and maintenance treatment protocols while avoiding conditioning chemotherapy.
“We are thrilled with the preliminary results from the low-dose groups of our FT522 Phase 1 trial in B-cell lymphoma. We have identified a favorable safety profile, instances of complete responses alongside conditioning chemotherapy, and the promising potential of our ADR-enhanced CAR NK cell product to maintain functional presence and selectively eliminate pathogenic CD19+ B cells without requiring conditioning chemotherapy,” stated Scott Wolchko, President and CEO of Fate Therapeutics. “We are confident that these findings strongly support our distinctive therapeutic approach in treating autoimmunity, and we are eager to evaluate FT522 clinically as an additional option to standard induction and maintenance therapies without the need for conditioning chemotherapy.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
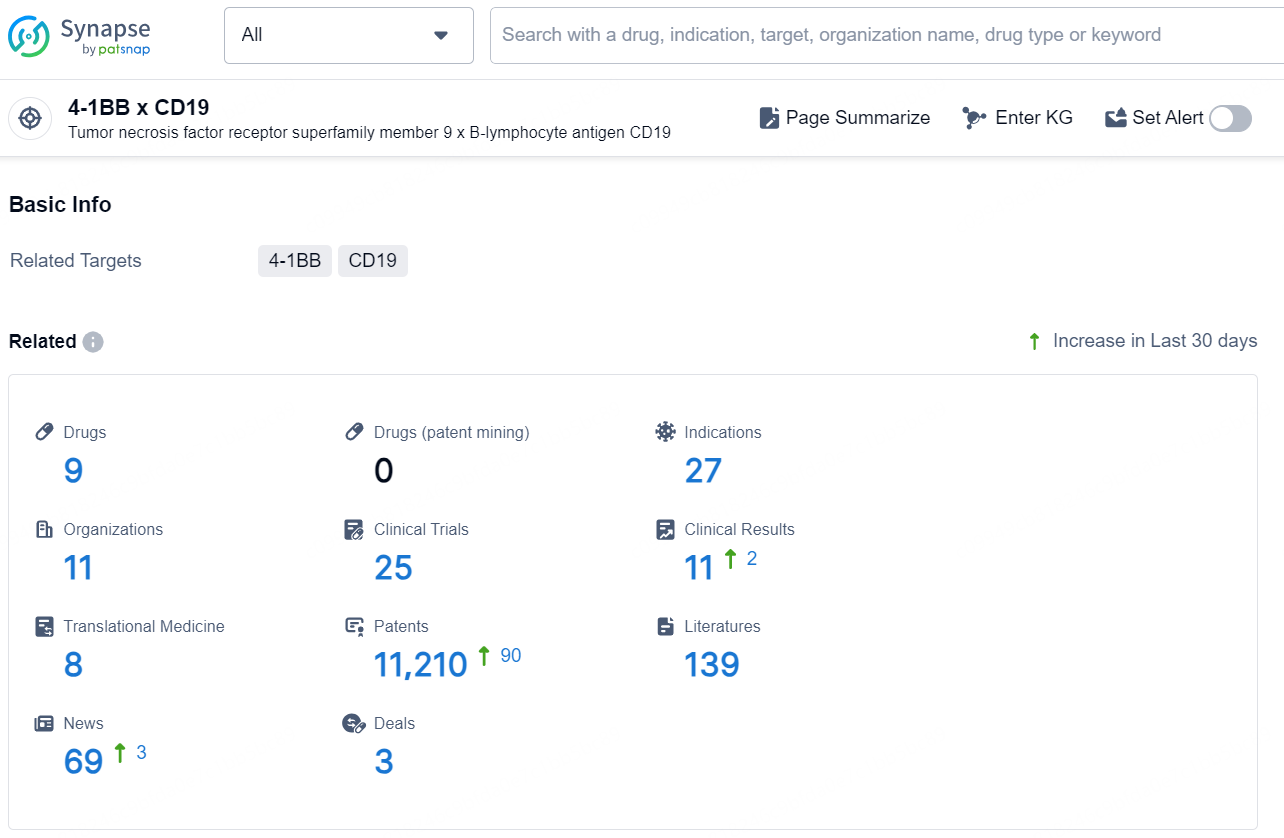
According to the data provided by the Synapse Chemical, As of November 20, 2024, there are 9 investigational drugs for the 4-1BB x CD19 target, including 27 indications, 11 R&D institutions involved, with related clinical trials reaching 25, and as many as 11210 patents.
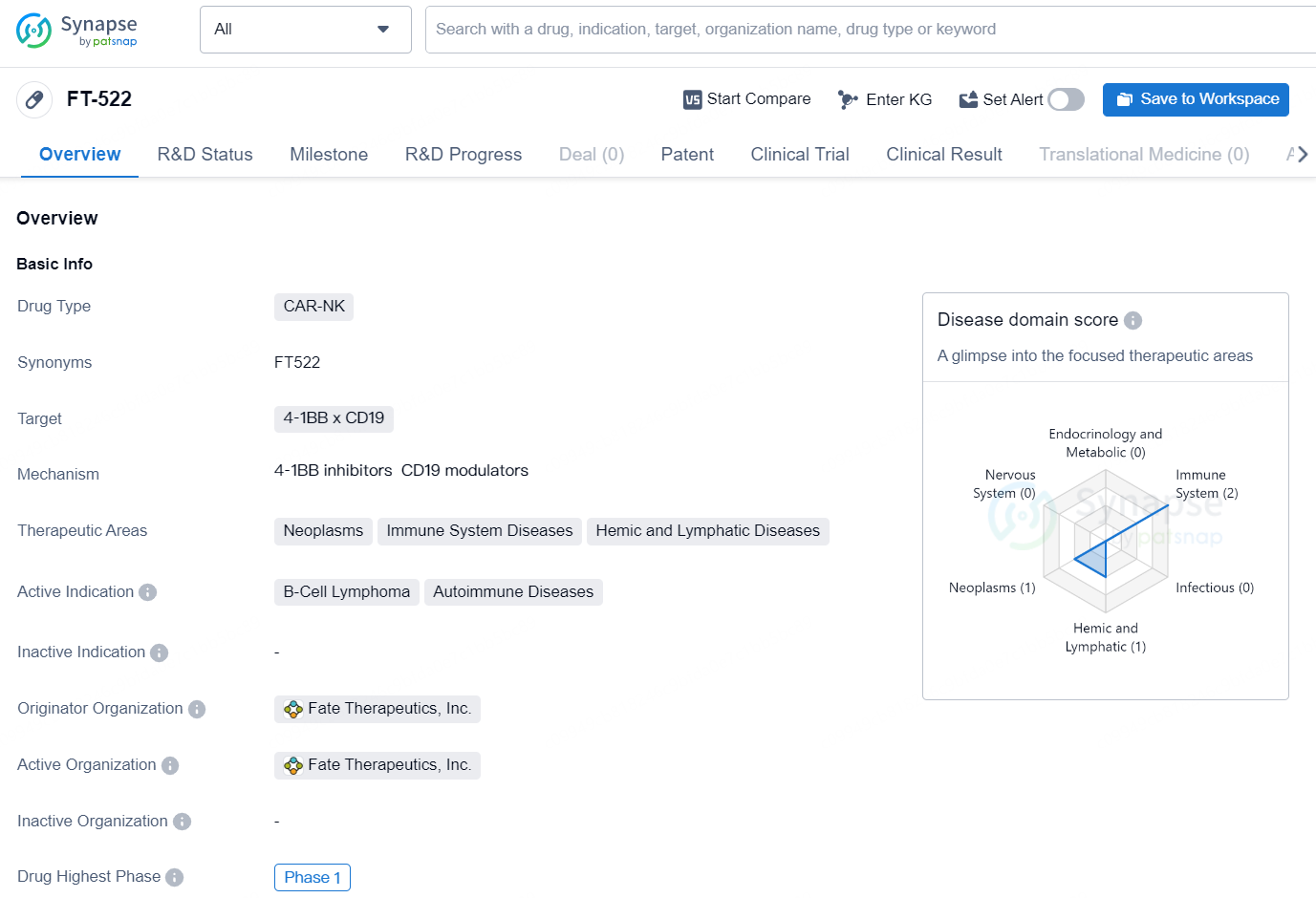
FT-522 is a CAR-NK type of drug developed by Fate Therapeutics, Inc. The drug targets the 4-1BB x CD19 and is intended for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. The active indications for this drug include B-Cell Lymphoma and autoimmune diseases.