Moderna RSV Vaccine: A Glimmer of Hope in a Billion-Dollar Race?
On August 21st, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO™ for active immunization in pregnant women of 32 to 36 weeks gestation in order to prevent Lower Respiratory Tract Disease (LRTD) and severe LRTD caused by RSV infection in infants under six months old from birth. This is the first and only time the FDA has approved a maternal vaccine.
In 2023, there was significant progress in the field of RSV vaccines.
On May 4th, GSK announced that the FDA approved arexvy for preventing respiratory syncytial virus (RSV) induced lower respiratory tract disease (LRDT) in individuals above 60 years old. On June 6th, Pfizer announced that its RSV vaccine, Abrysvo, got FDA approval for preventing RSV-LRDT in people over 60 years of age. On July 17th, The US Food and Drug Administration (FDA) approved a monoclonal antibody, Nirsevimab, developed by Sanofi and AstraZeneca, for preventing RSV-LRDT in all infants. It is the first long-term prevention measure for RSV in all infants.
With the consecutive approval of RSV vaccines, 2023 has been referred to as the "year of commercialization of RSV vaccines".
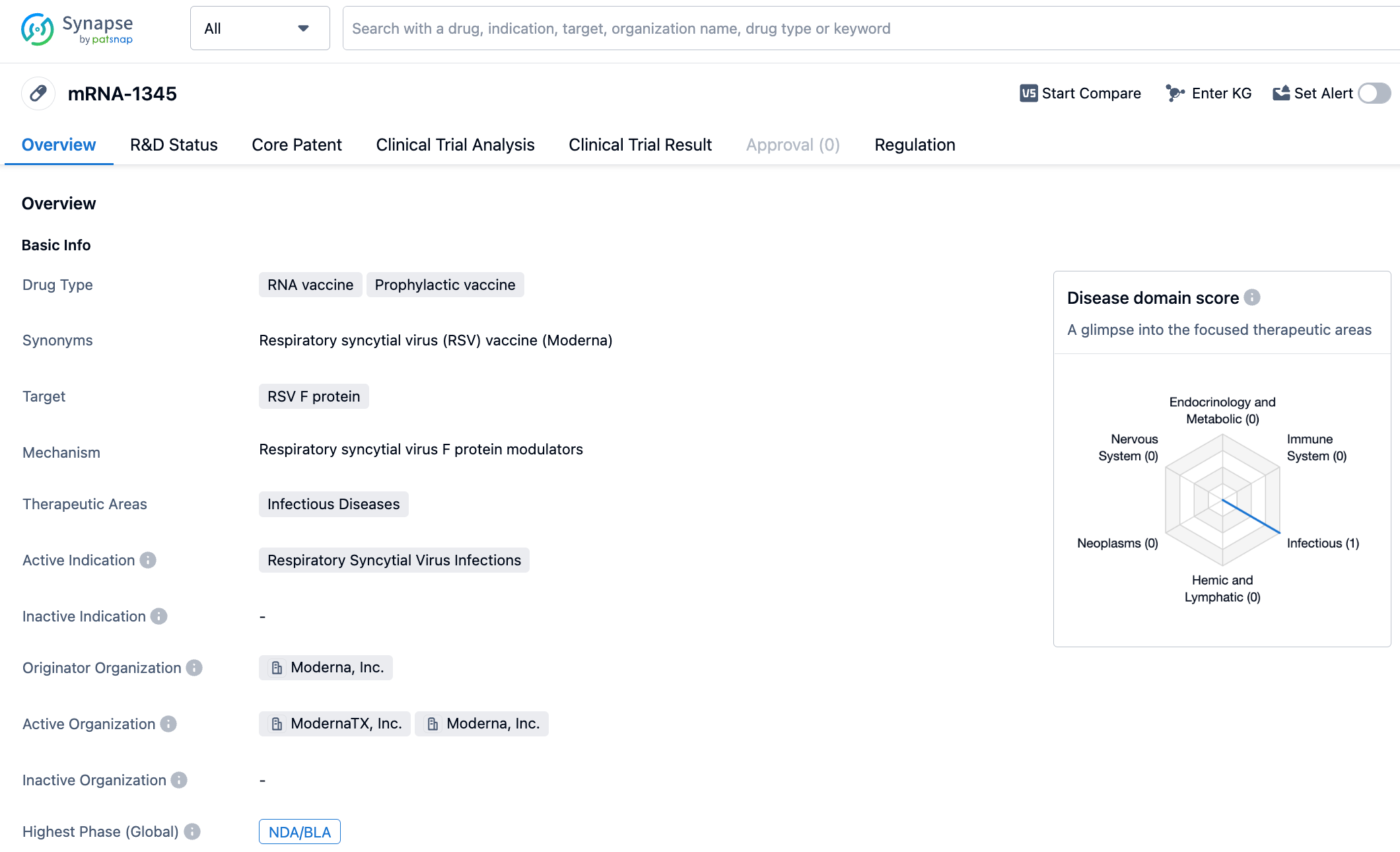
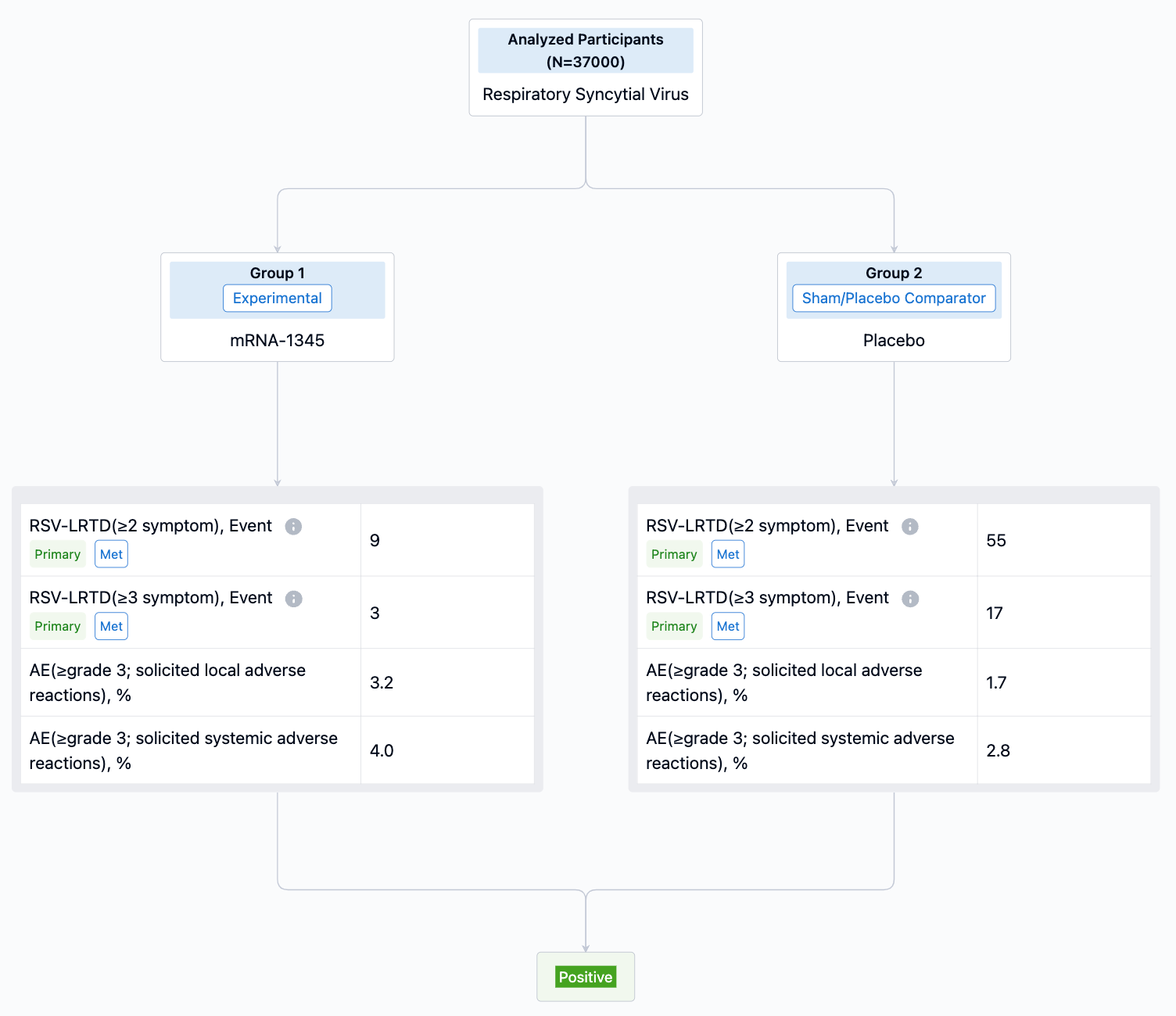
Besides the aforementioned pharmaceutical giants, Moderna's RSV vaccine based on mRNA technology is also expected to be approved soon. On July 5th, Moderna announced that it had submitted applications to several global regulatory bodies for its RSV vaccine mRNA-1345, including a rolling submission of Biologics License Application to the FDA. Earlier, Moderna announced data showing that mRNA-1345 achieved the primary endpoint in Phase III clinical trials of preventing RSV infection in elderly individuals, with a protection rate of 83.7% for those with two or more symptoms of RSV-LRTD, and 82.4% efficacy for those with three or more symptoms.
History of RSV Vaccine Development
Respiratory Syncytial Virus (RSV) is a virus that infects the respiratory tract and is common in infants and the elderly, especially those less than six months old, immunocompromised or suffering from chronic diseases. RSV can cause childhood respiratory diseases, pneumonia, and bronchitis, making it one of the most common causative agents of pediatric respiratory infections worldwide. Clinical manifestations of RSV are complex, including fever, cough, runny nose, wheezing, and in severe cases, it can trigger life-threatening symptoms such as shortness of breath, respiratory arrest, and cyanosis.
The genomic structure of RSV is non-segmented single-stranded negative-strand RNA, with a full length of approximately 15.2kb, encoding 11 proteins, including 8 structural proteins (F, G, M2-1, M2-2, SH, N, P, L) and 3 nonstructural proteins (NS1, NS2, NS3). The fusion protein (F) and the attachment protein (G) are the two major envelope glycoproteins. F is a typical type I glycoprotein that, after being cleaved into F1 and F2 by cellular proteases, possesses biological activity and can cause the virus envelope to fuse with the host cell membrane to form multinucleated giant cells. The G protein is a type II glycoprotein and can bind to host cell membrane receptors, mediating the entry of the virus into the host cell. Compared to protein G, protein F shows less variability and is relatively stable, making it an important target for drug development.
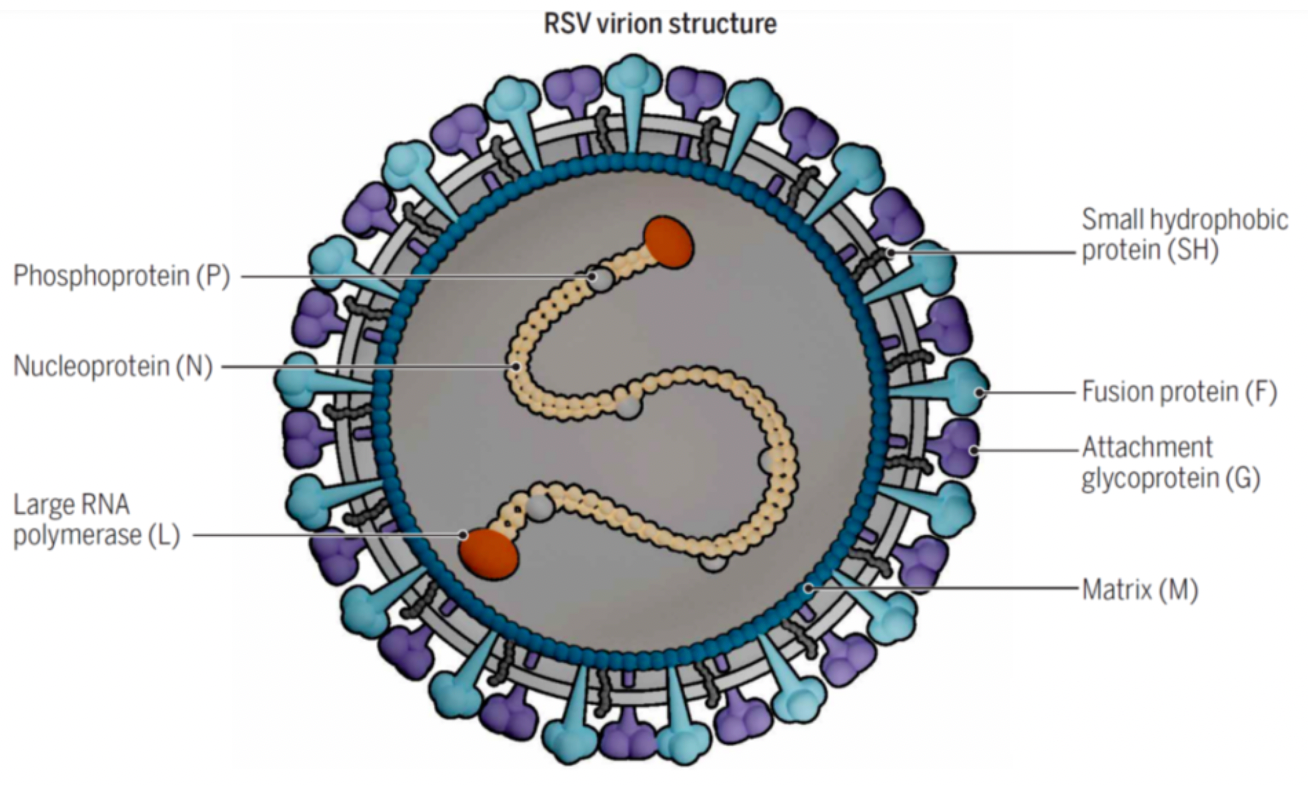
As early as 1956, the RSV virus was isolated from the respiratory tract of chimpanzees, but until 2013, RSV vaccines were all designed and developed against post-fusion F protein (Post-F). This may explain the failures of the vaccine. Thereafter, the strategies and types of vaccine development rapidly increased.
In order to stabilize the shape of pre-F protein, researchers added a key chemical bond in the protein, acting like double-sided adhesive tape, to keep the protein folded into the pre-F shape.
It is these improvements against pre-F protein that has made pre-F antigen vaccines possible, bringing the success of RSV vaccines one step closer.

Moderna RSV Vaccine Layout
Moderna's RSV vaccine, mRNA-1345, is an mRNA vaccine that encodes the virus's stable pre-fusion F Glycoprotein, which can elicit a better neutralizing antibody response compared to its post-fusion state. The vaccine uses the same lipid nanoparticles as Moderna's COVID-19 vaccine. The F Glycoprotein is located on the surface of the virus and aids the virus in infecting host cells. It exists in two states: pre-fusion and post-fusion, with the pre-fusion conformation being an important target for effective neutralizing antibodies, and the protein sequences of RSV-A and RSV-B subtypes are very similar.
Previously, the U.S. FDA has granted mRNA-1345 Fast Track designation and Breakthrough Therapy designation to help prevent RSV in adults over 60 years old.
In January 2023, Moderna released phase three clinical data of mRNA-1345, indicating that the study met its primary endpoint with an efficacy of 83.7% in preventing Respiratory Syncytial Virus (RSV) associated lower respiratory tract diseases in individuals aged 60 and over. In terms of safety, the drug was well-tolerated with no identified safety concerns. The most common adverse effects were mild or moderate, including injection site pain, fatigue, headaches, muscle pain, and joint pain. In the same month, mRNA-1345 received a breakthrough therapy designation from the FDA for the prevention of RSV-associated lower respiratory tract diseases (RSV-LRTD) in adults aged 60 and over. This vaccine is expected to be approved and hit the market this year, providing a new treatment option for RSV infections.
After over six decades in development, the RSV vaccine has finally seen the light of day, offering a breath of fresh air to this market valued at billions of dollars.