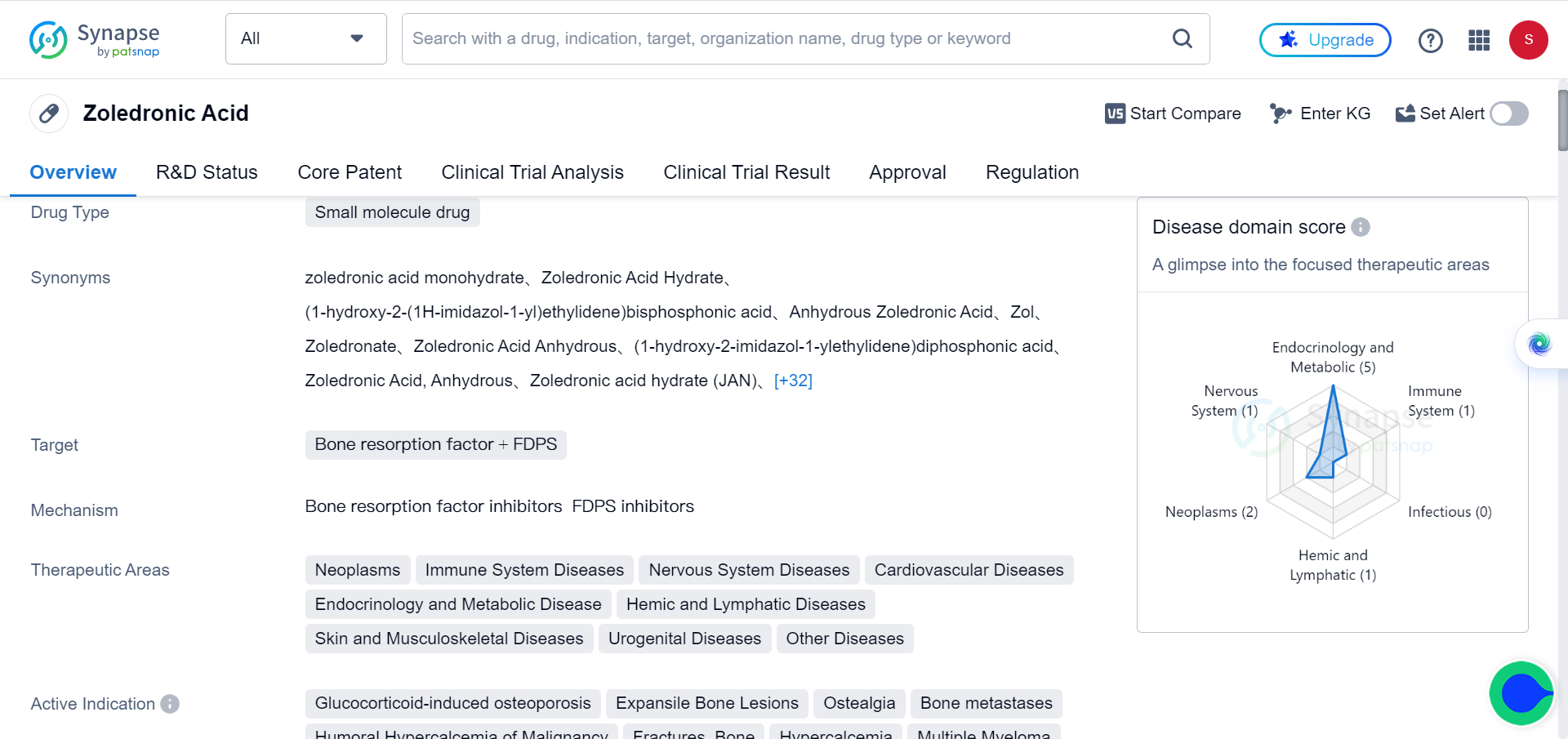
Zoledronic Acid holds the key in Fighting Aging-Related Diseases
Aging is a major risk factor for multiple age-related diseases, including sarcopenia, cardiovascular disease, cognitive disorders, arthritis, respiratory disorders, osteoporosis, and frailty. While treatments exist for individual diseases, the overall disease burden is expected to increase markedly with the growing elderly population, projected to more than double by 2050.
Cellular senescence, characterized by irreversible growth arrest and secretion of pro-inflammatory factors (senescence-associated secretory phenotype, SASP), contributes to aging and related diseases. Senescent cell accumulation is linked to common age-related diseases, and clearance extends healthspan in mice. However, no therapies currently target senescence in humans.
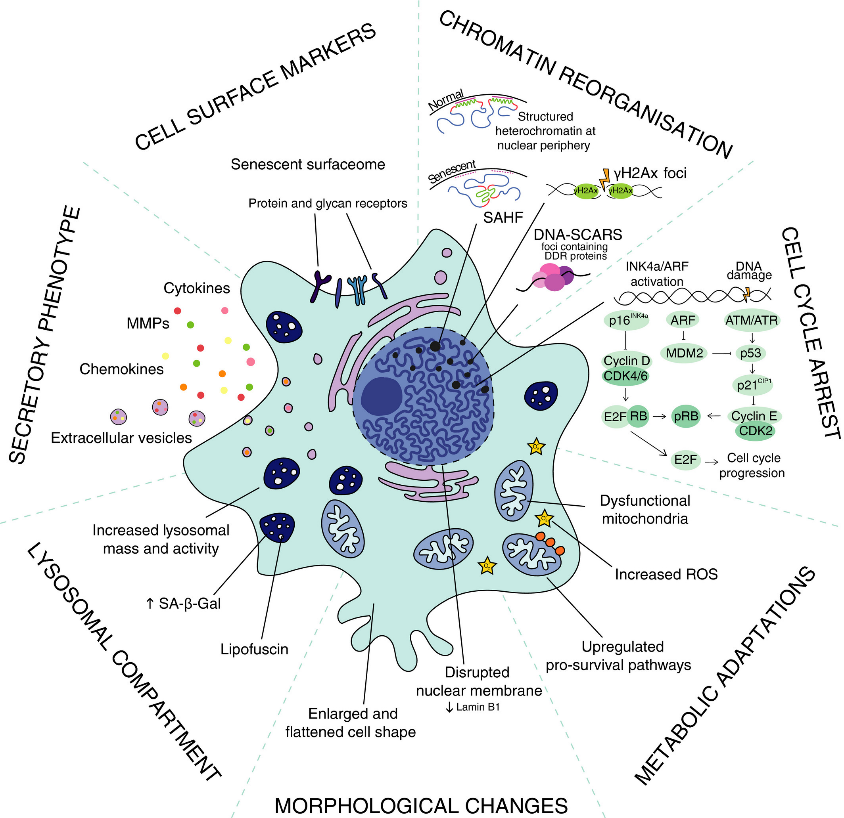
Zoledronic acid against cellular senescence
A recent study published in journal Aging has shed light on the potential anti-aging effects of zoledronic acid, a commonly prescribed bisphosphonate. The research conducted by an international team of scientists from leading institutions explored the impact of zoledronic acid on cellular senescence and the senescence-associated secretory phenotype (SASP), which are known to contribute to various age-related diseases.
Zoledronic acid may target senescence. It improves bone mineral density and reduces fractures. It has also been associated with reduced mortality, cancer, and cardiovascular disease in some studies. Animal studies show it increases lifespan in progeria mice, inhibits tumors, protects cells from radiation, and improves muscle after chemotherapy - similar to senescent cell clearance. However, it is unclear if zoledronic acid's skeletal or extra-skeletal effects relate to senolytic or senomorphic activity. Thus, the researchers evaluated zoledronic acid's potential effects on modulating cellular senescence using in vitro, in vivo, and in silico approaches.
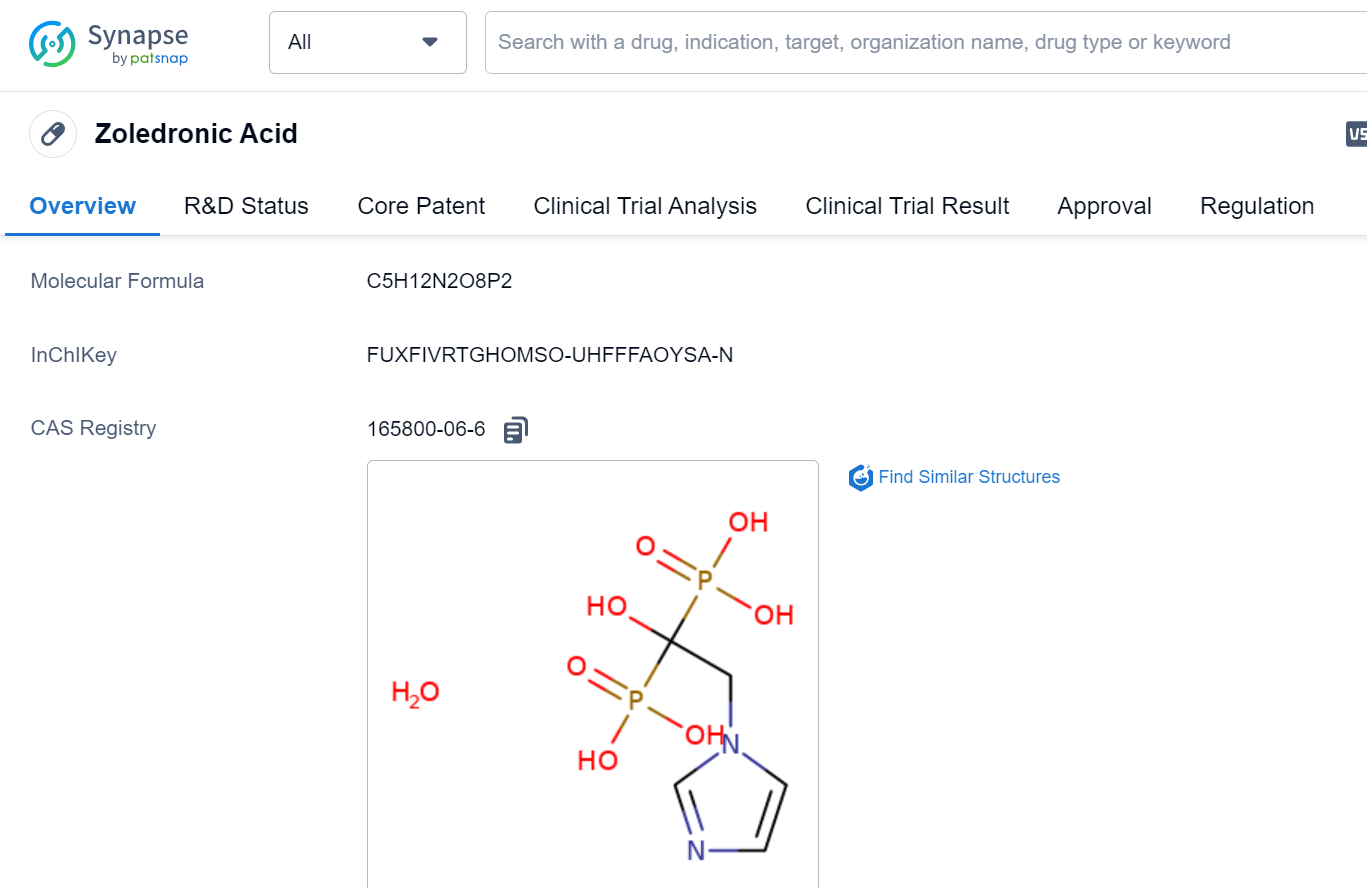
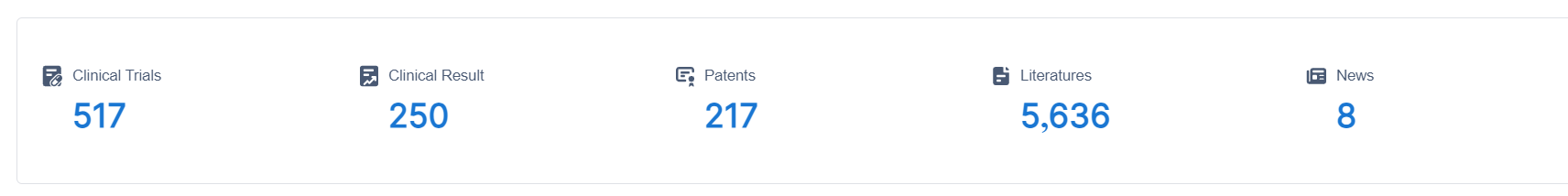
The study, led by Parinya Samakkarnthai from the Mayo Clinic in the USA and Dominik Saul from Eberhard Karls University Tübingen in Germany, utilized both in vitro and in vivo experiments to investigate the effects of zoledronic acid on senescent cells.
In the initial in vitro experiments, human lung fibroblasts and DNA repair-deficient mouse embryonic fibroblasts were exposed to zoledronic acid. The results revealed that the compound effectively killed senescent cells while having minimal impact on non-senescent cells. This indicated the potential senolytic (senescent cell-killing) properties of zoledronic acid.
Moving to in vivo studies, aged mice were treated with zoledronic acid or a control vehicle for a period of 8 weeks. The researchers found that zoledronic acid significantly reduced the levels of SASP factors in the mice, including CCL7, IL-1β, TNFRSF1A, and TGFβ1. Additionally, the treated mice exhibited improved grip strength, suggesting a positive effect on physical function.
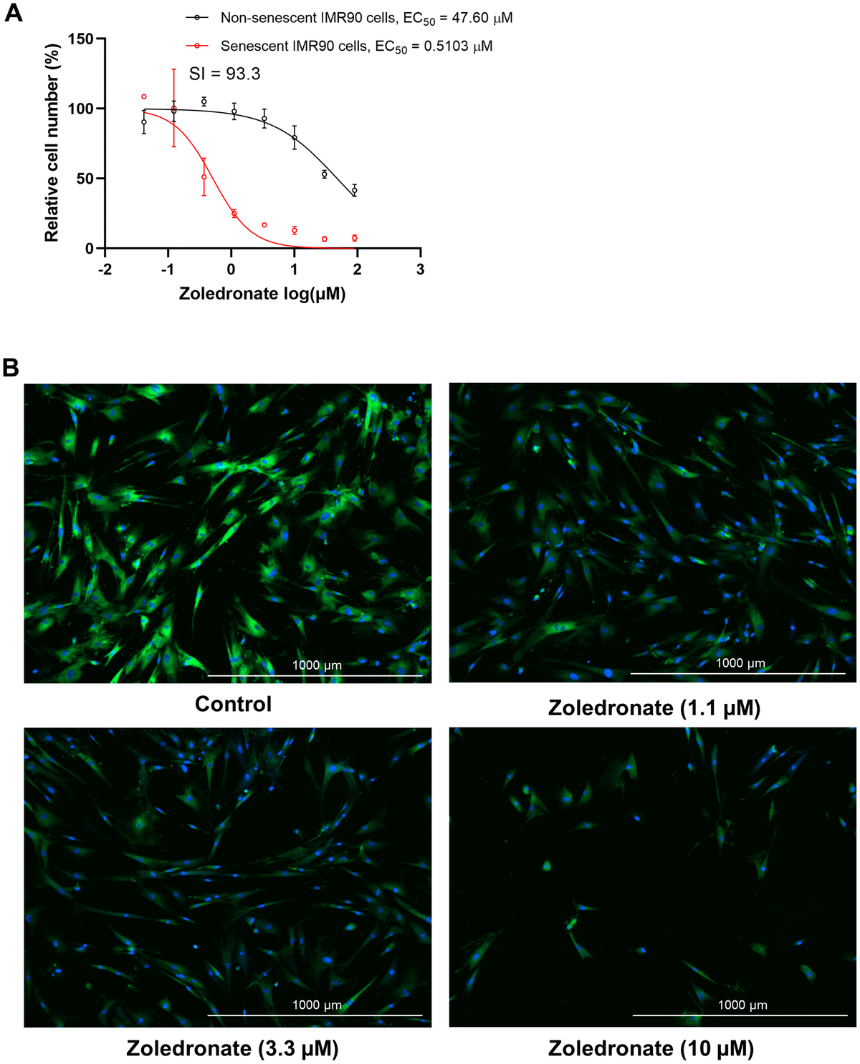
To further investigate the underlying mechanisms, the team analyzed publicly available RNA sequencing data from pre-osteoclastic cells isolated from mice treated with zoledronic acid. The analysis demonstrated a significant downregulation of senescence/SASP genes, reinforcing the potential senolytic or senomorphic (inhibition of SASP secretion) actions of the compound.
To confirm zoledronic acid's impact on specific cell populations, single-cell proteomic analysis (cytometry by time of flight - CyTOF) was employed. The analysis revealed a notable reduction in the number of pre-osteoclastic cells and decreased protein levels of p16, p21, and SASP markers within these cells. Importantly, other immune cell populations remained unaffected by the treatment.
The findings of this study provide compelling evidence that zoledronic acid possesses senolytic effects in vitro and modulates senescence/SASP biomarkers in vivo. These results highlight the potential of zoledronic acid and other bisphosphonate derivatives as senotherapeutic agents for combating age-related diseases. However, further research and clinical trials are needed to fully understand the therapeutic potential of zoledronic acid in combating age-related diseases in humans.
The study's findings open up new avenues for exploring senescence-targeting therapies and offer hope for a future where age-related diseases can be effectively prevented and treated.
Improves Osteoporosis by Acting on Osteoclasts
Zoledronic acid is already widely used to treat osteoporosis and skeletal complications, its additional benefits in reducing mortality, cancer risks, and cardiovascular diseases have been observed in separate studies. In particular, previous studies have highlighted the potential of zoledronic acid in effectively combating osteoporosis by targeting osteoclasts, the cells responsible for bone resorption.
Zoledronic acid has emerged as a leading anti-osteoporotic drug due to its remarkable efficacy and treatment compliance. Notably, intravenous administration of zoledronic acid offers a therapeutic effect for up to one year after infusion. The study explores the mechanisms through which zoledronic acid improves osteoporosis and establishes that zoledronic acid acts on osteoclasts to inhibit their differentiation, induce apoptosis, and enhance bone density. The canonical receptor activator of nuclear factor-κB ligand (RANKL) pathway and the non-canonical Wnt pathway are identified as the primary pathways through which zoledronic acid inhibits osteoclast differentiation. Additionally, the study suggests that farnesyl pyrophosphate synthase (FPPS), reactive oxygen species (ROS), and ferroptosis are closely associated with zoledronic acid -induced osteoclast apoptosis.
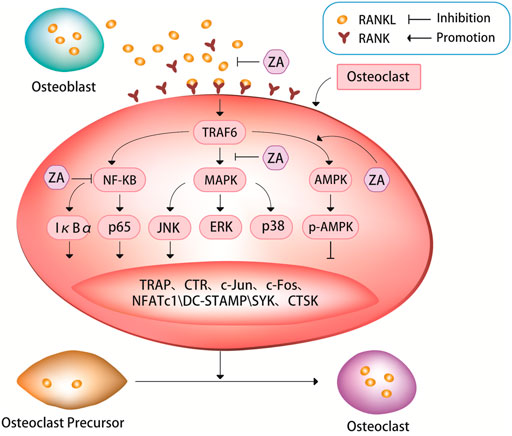
Osteoporosis, characterized by decreased bone mass and increased bone voids, results from an imbalance between osteoclastic bone resorption and osteoblastic bone formation. Consequently, inhibiting osteoclast differentiation and promoting osteoclast apoptosis are key strategies for treating osteoporosis.
zoledronic acid, a third-generation bisphosphonate with high affinity for bone tissues, effectively suppresses osteoclastic bone resorption. By inhibiting the activity of FPPS in the mevalonate pathway, zoledronic acid selectively targets osteoclasts, preventing the breakdown and absorption of bone.
The findings of this study hold significant promise for the treatment of osteoporosis. By elucidating the intricate relationship between zoledronic acid and osteoclasts, the researchers hope to provide valuable insights for the development of novel therapeutic approaches.
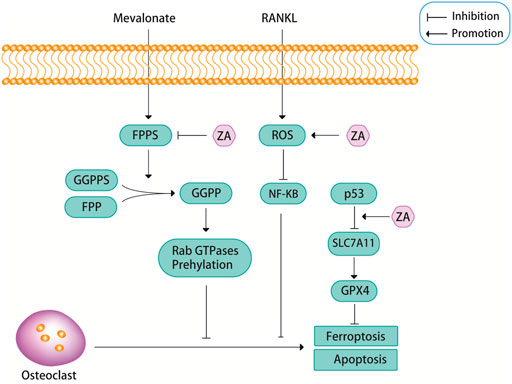
Osteoporosis is a pressing global health concern, affecting millions of individuals, particularly the elderly. With current estimates suggesting that one in three women and one in five men over the age of 50 will experience osteoporotic fractures, the need for effective treatments is paramount.
As the understanding of the role of osteoclasts in bone resorption continues to evolve, researchers are optimistic that novel interventions targeting these cells, such as zoledronic acid, may revolutionize the management of osteoporosis.
References:
1.Samakkarnthai, P. et al. (2023). In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging, 15(9), 3331-3347. DOI: 10.18632/aging.204701
2.Wang B, Zhan Y, Yan L and Hao D (2022) How zoledronic acid improves osteoporosis by acting on osteoclasts. Front. Pharmacol. 13:961941. doi: 10.3389/fphar.2022.961941