Molecules Transform Repressor to Activator Enabling Cancer Cell Apoptosis
In a recent study, researchers from Stanford University in the US have made a significant discovery in the field of cancer treatment. They have identified a novel class of molecules called transcriptional/epigenetic chemically induced proximity (transcriptional/epigenetic CIP, TCIP), which has the potential to induce cell death in approximately 50% of cancers. The research findings were published online in the journal Nature, under the title "Rewiring cancer drivers to activate apoptosis."
The double-headed molecule
Chemical-induced proximity (CIP) plays a crucial role in various biological regulations. This includes post-translational modifications like phosphorylation and methylation, as well as allosteric processes that create platforms or frameworks for facilitating protein interactions. To understand the biological significance of CIP, researchers have used genetically tagged proteins to investigate the impact of proximity and conformational changes. Recent advancements have also led to the development of small molecules that utilize CIP to target proteins to the proteasome (PROTACS) or induce stabilization. These studies have demonstrated that CIP is involved in signal transduction, transcription, and can replicate several steps in these processes. In the nucleus, induced proximity is essential for activating transcription and regulating epigenetic repression or activation.
A notable example highlighting the role of CIP is the activation of death receptors, such as the FAS receptor (FASR or CD95), which eliminates autoreactive cells in the immune system. Through induced proximity of their death domains or the initiator caspases, apoptosis is rapidly and effectively initiated. This discovery shows that even a highly regulated process like programmed cell death can be triggered through CIP. This suggests that components within these signal transduction and transcriptional pathways could be interconnected or rewired to induce apoptosis in cancer cells.
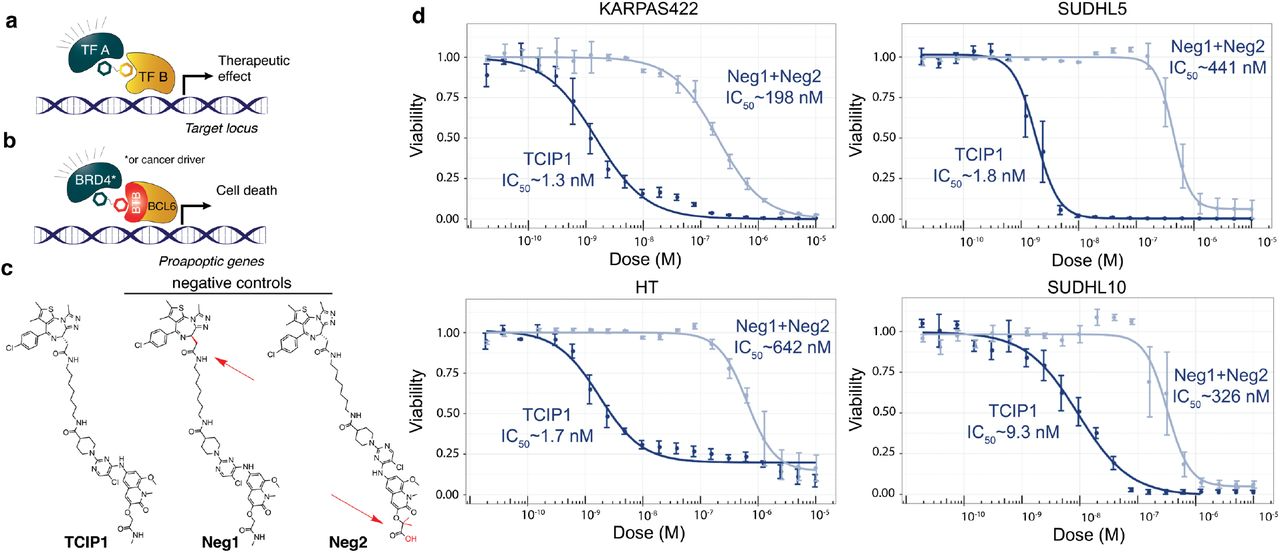
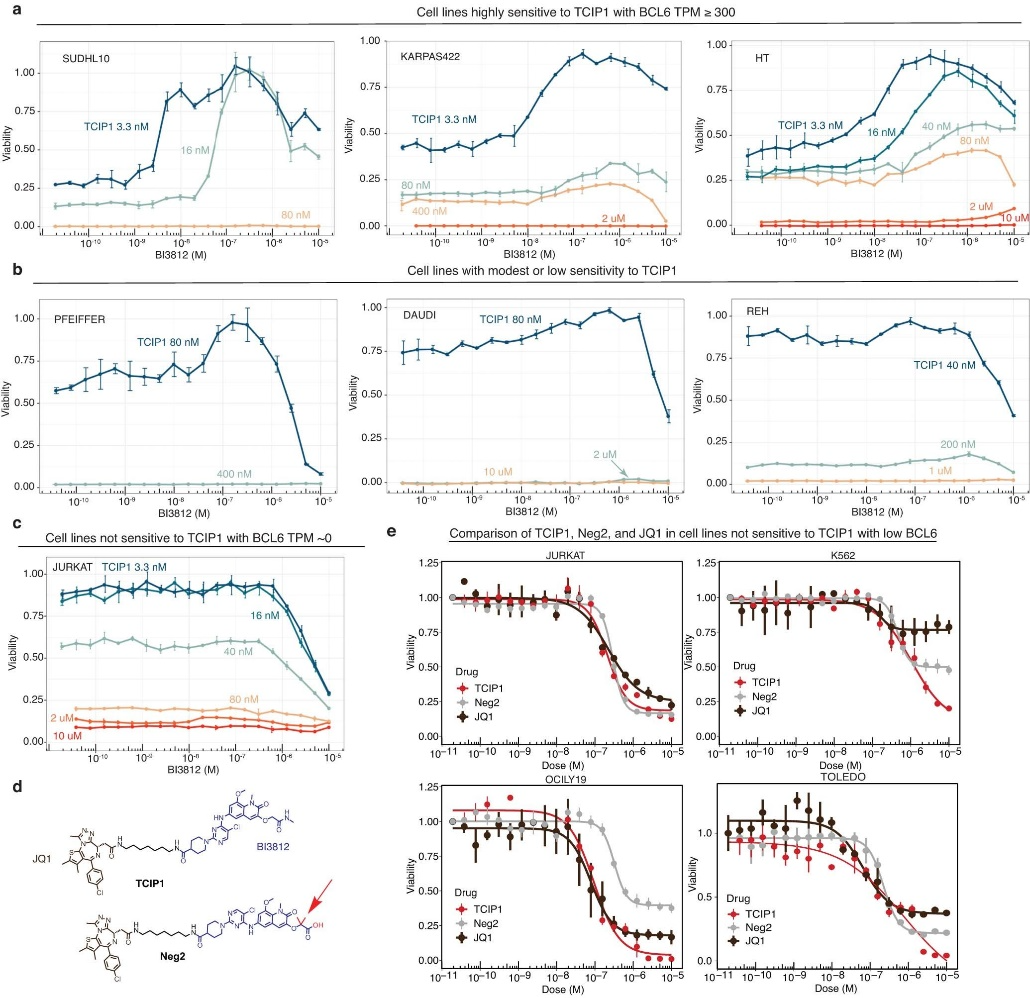
The researchers designed small molecules that can effectively bring together specific transcriptional suppressors and activators. Among the molecules they created, TCIP1 demonstrated the highest efficacy. TCIP1 functions by linking a molecule that binds to BCL6 with a molecule that binds to the transcriptional activator BRD4.
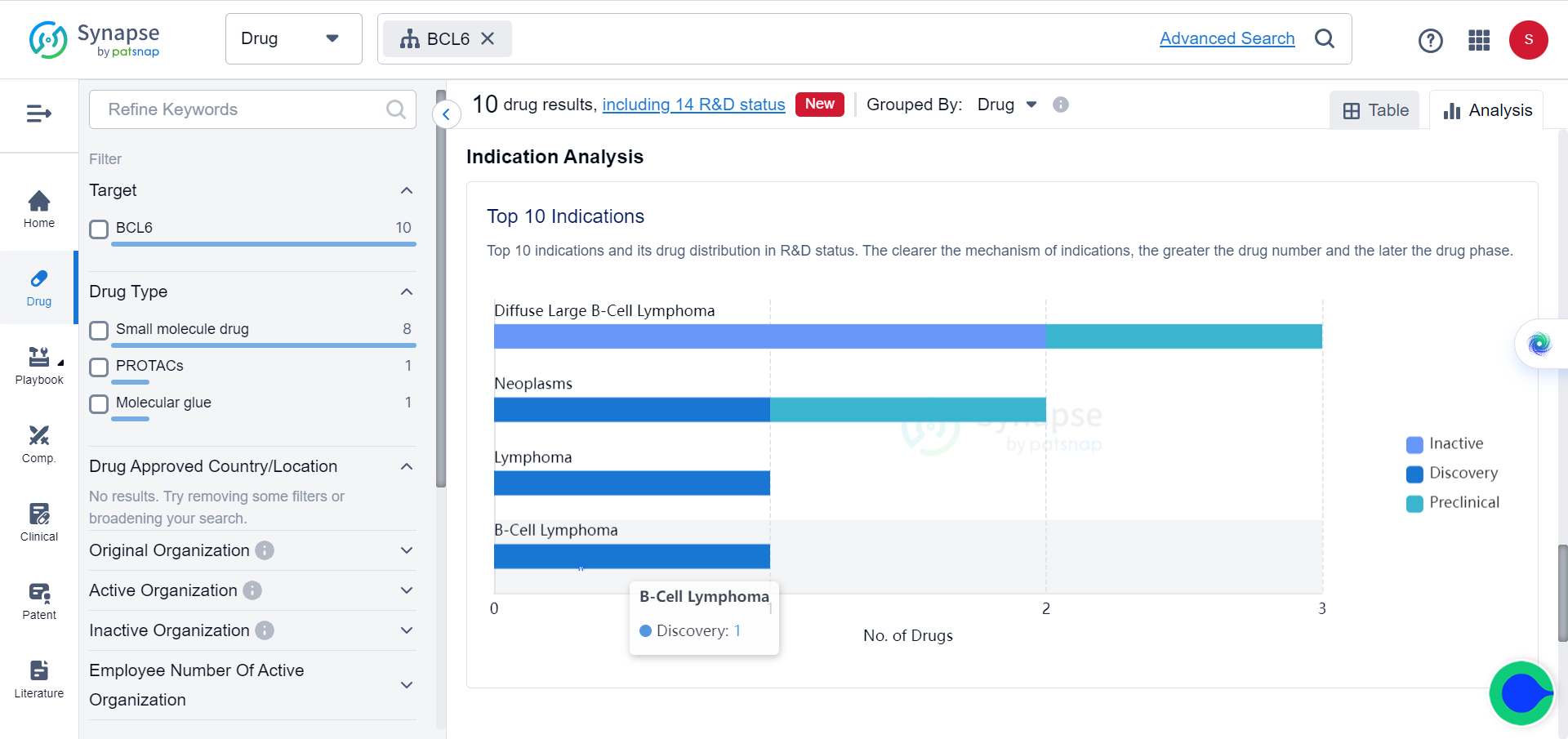
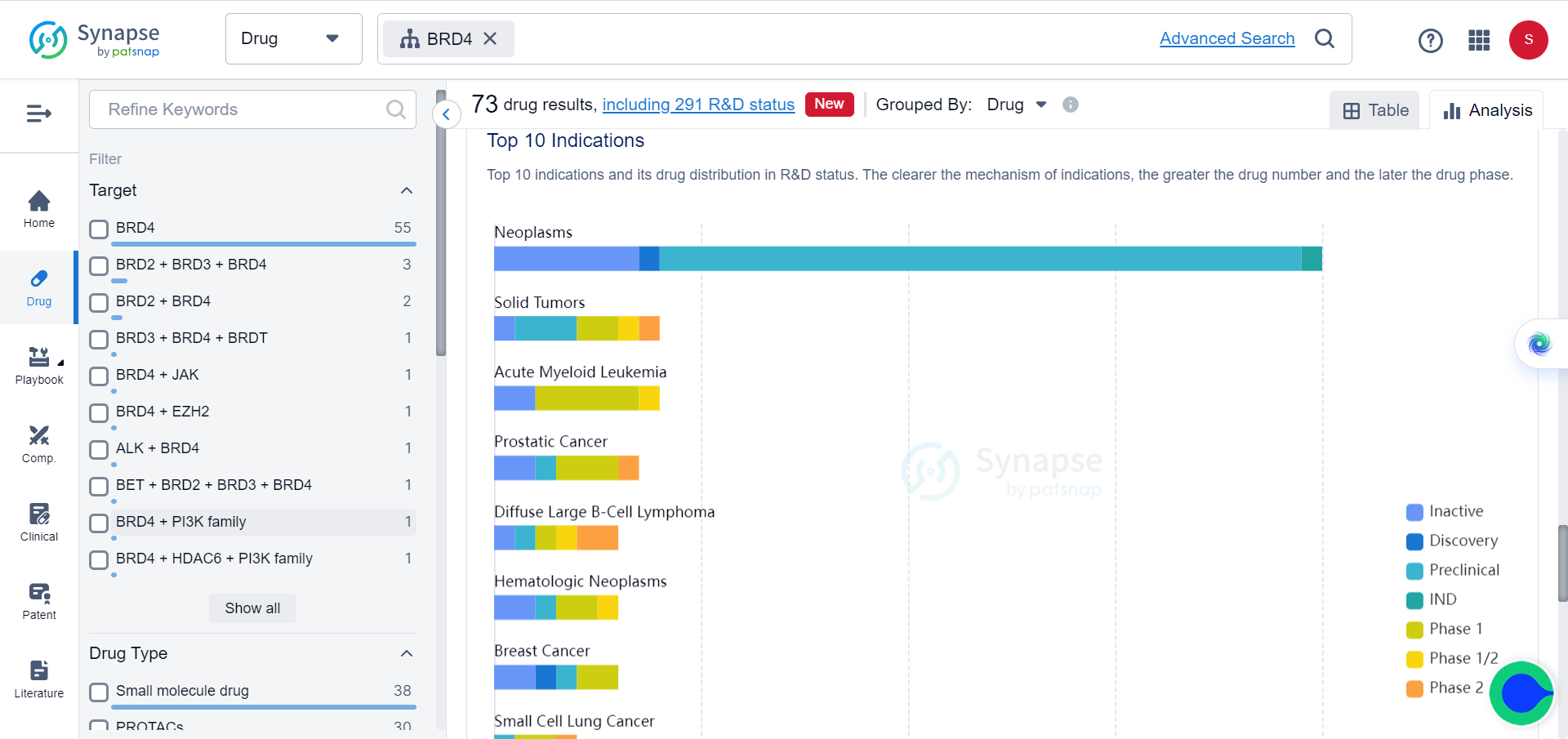
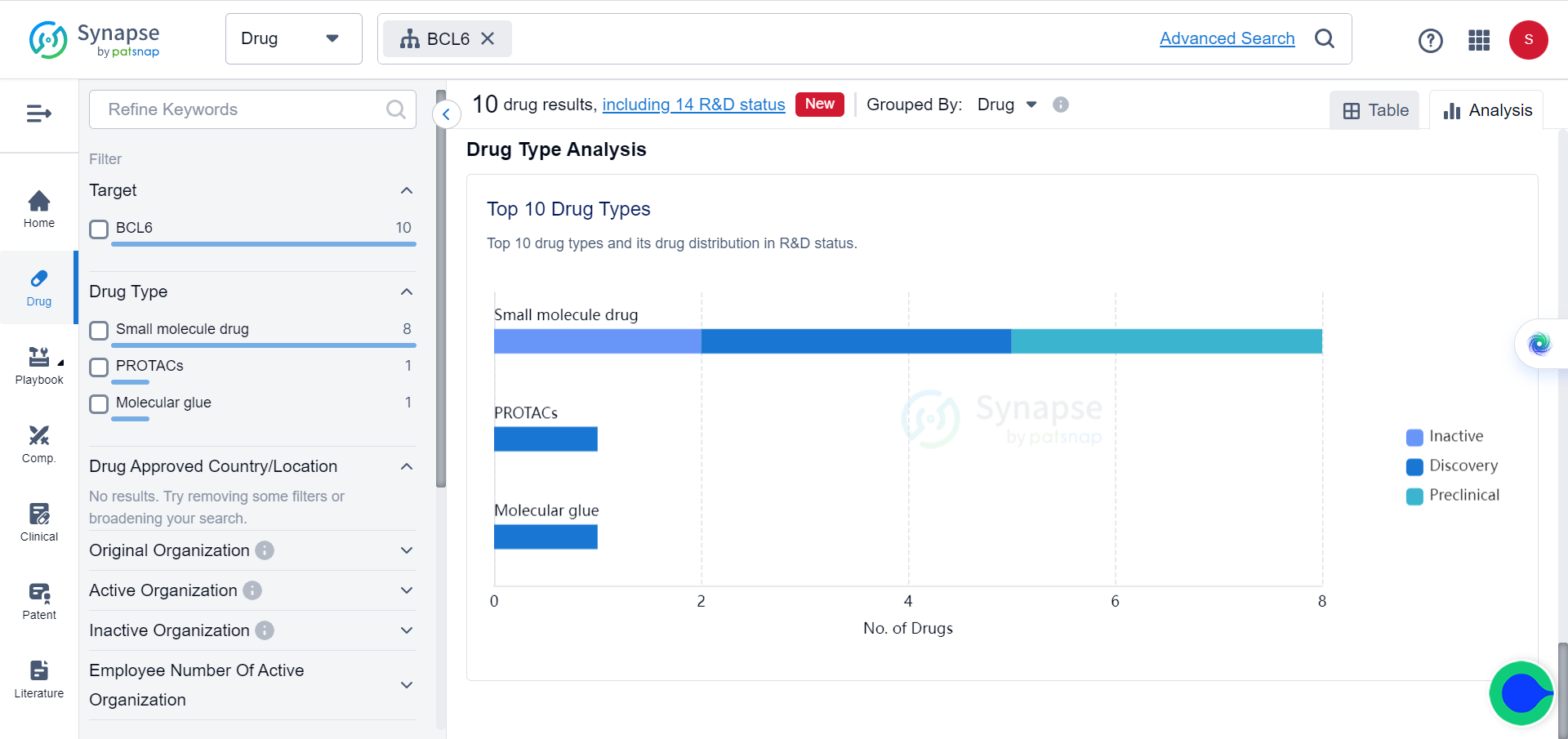
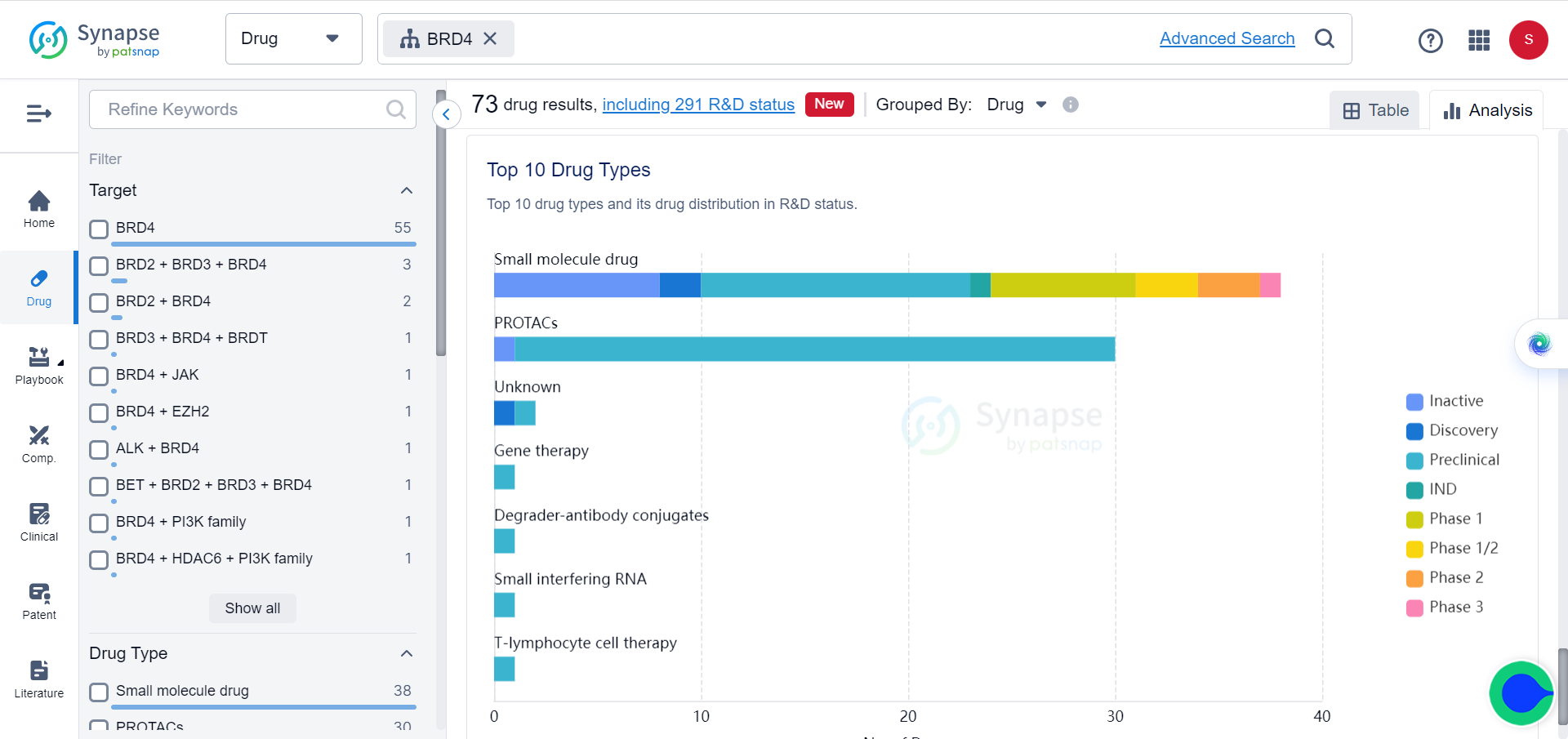
In Synapse database, although BCL6 and BRD4 are already popular targets of multiple drugs, which mostly consist of small molecules and PROTACs, for a variety of tumors, none of these drugs target them simultaneously.
Cancer cells become malignant when they disregard signals from surrounding healthy tissue to cease growth and initiate cell death. Although the apoptosis pathway still exists in these cells, it is actively blocked in certain types of cancer. The transcription factor BCL6 binds to the promoters of pro-apoptotic genes and suppresses their expression through epigenetic mechanisms.
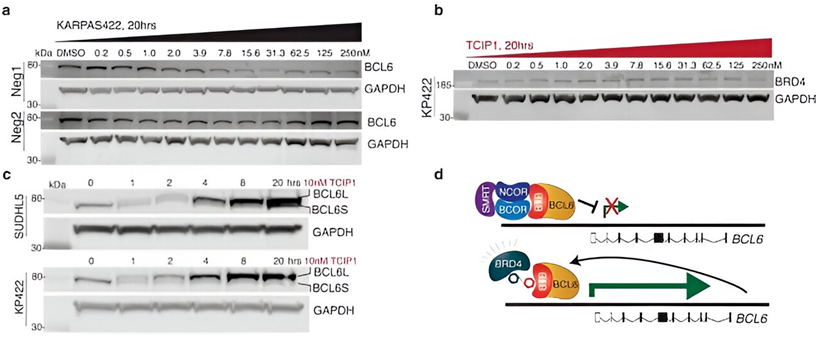
By combining a BCL6-binding molecule with a BRD4-binding molecule, the researchers developed TCIPs. Transcriptional activators like BRD4 have normal activity in cancer cells, promoting rapid cancer growth and proliferation. When coupled with BCL6, BRD4 activates the expression of any genes that BCL6 is bound to, including pro-apoptotic genes that cancer cells try to suppress.
TCIP1 successfully induced cell death in large B cell lymphoma cell lines, even those that were resistant to chemotherapy and harbored TP53 mutations. Moreover, its effects were specific to certain cell types and tissues, with apoptosis occurring within 72 hours.
Addressing potential safety concerns
BCL6 is crucial for the lymphatic system, as mice lacking the BCL6 gene die due to complex inflammatory reactions. BRD4 is closely associated with genomic function and stability in various processes. This study addresses concerns about utilizing the expression of these important genes and their potential impact on off-target healthy tissue.
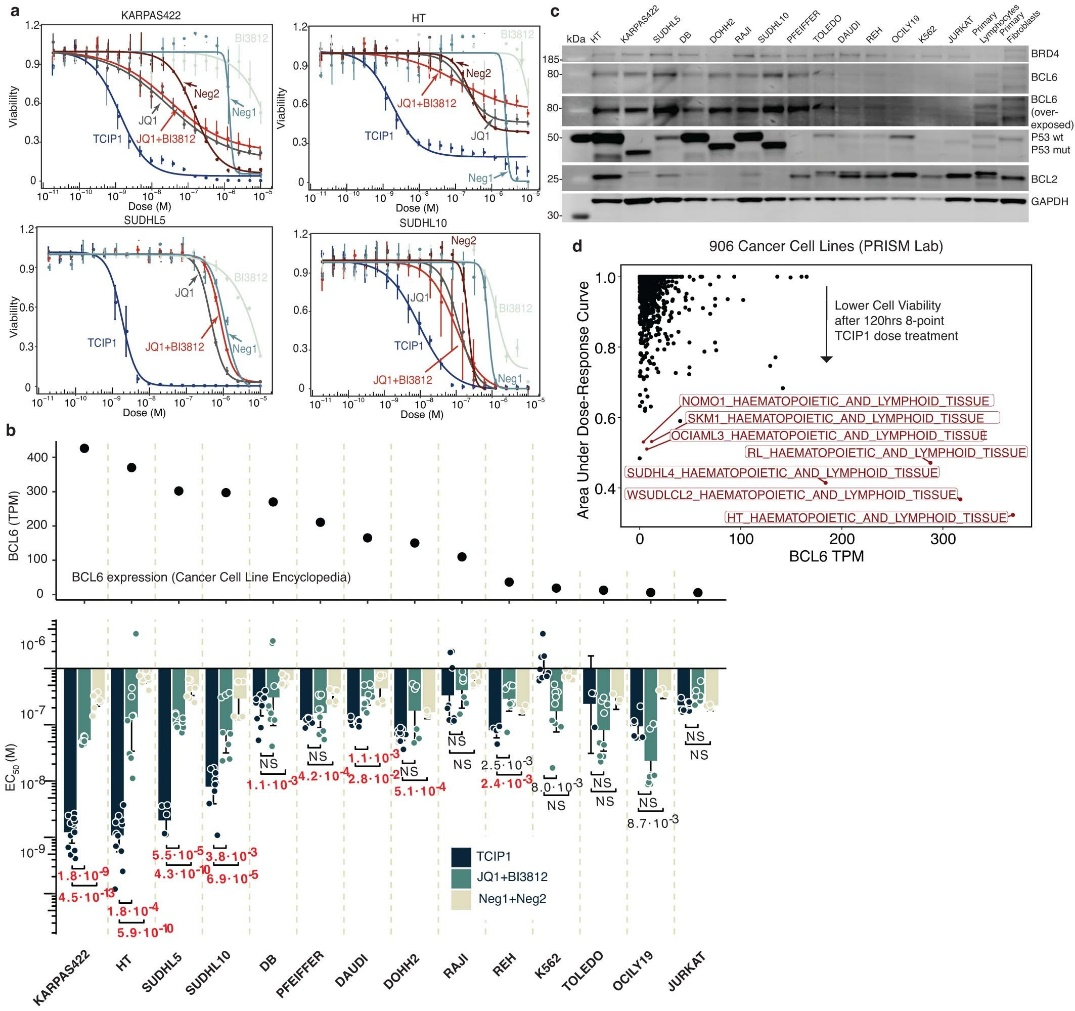
TCIP1 acts in an environment-specific manner, requiring the presence of both BRD4 and BCL6 expression to bind to them. Furthermore, it works at concentrations that occupy only a small fraction of total BCL6 molecules.
In mouse models, TCIPs demonstrated no toxicity to cells lacking BCL6. The TCIPs designed by the researchers had no effect on triple-negative breast cancer cell lines lacking BCL6.
TCIP1 induced significant transcriptomic changes in the spleen, including the upregulation of FOXO3, indicating activity in the target cancer cells. Despite these alterations, TCIP1 was well-tolerated, with no adverse reactions, significant weight differences in mice, or visible abnormalities such as inflammatory infiltrates or apoptosis.
TCIPs function by rewiring only a portion of the cancer-driving molecules within each cell. At the concentrations used in this study, TCIP1 increased BRD4 at BCL6 sites by 1.5-fold without disrupting normal activity at other sites. This demonstrates that TCIPs have the potential to activate genes and exert cytotoxic effects within cancer cells, making them a promising candidate for therapeutic development. However, further research is necessary to assess their safety.
The researchers believe that the gene-activating capabilities of small molecule TCIPs could have broad applications in biology and medicine. These molecules could potentially be engineered to activate cell death pathways in senescent cells, induce the expression of therapeutic or haploinsufficient genes, activate the expression of novel antigens for human immunotherapy, or modulate gene expression in cells or organisms for synthetic biology applications.
While it is challenging to evaluate the therapeutic potential of a new approach at such an early stage of development, this method appears to be highly innovative and straightforward to obtain and utilize across various pathologies.

Reference
Gourisankar, S., Krokhotin, A., Ji, W. et al. Rewiring cancer drivers to activate apoptosis. Nature (2023).