Mozart Therapeutics Begins Phase 1 Trial of MTX-101 for Autoimmune Treatment
Mozart Therapeutics, a biopharmaceutical firm in the clinical stage devoted to creating CD8 Treg modulators for autoimmune disorders, has initiated dosing for the first group of participants in its pioneering clinical trial of MTX-101. MTX-101 is recognized as a novel CD8 Treg modulator and functions as an autoimmune checkpoint inhibitor.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Initiating the dosing process for the initial cohort in this Phase 1a/b clinical trial with MTX-101 is a pivotal achievement for Mozart Therapeutics, highlighting our entry into clinical stages and bringing us nearer to our objective of revolutionizing the treatment landscape by altering the trajectory of autoimmune diseases for every patient,” stated Katie Fanning, President and CEO of Mozart Therapeutics.
“In autoimmune disorders, CD8 Treg cells fail to identify and eradicate pathogenic or self-reactive CD4 T cells, leading to subsequent proinflammatory incidents and ongoing tissue damage. The goal of MTX-101 is to precisely address a root cause of immune system imbalance and reinstate immune equilibrium in autoimmune conditions,” added Ms. Fanning.
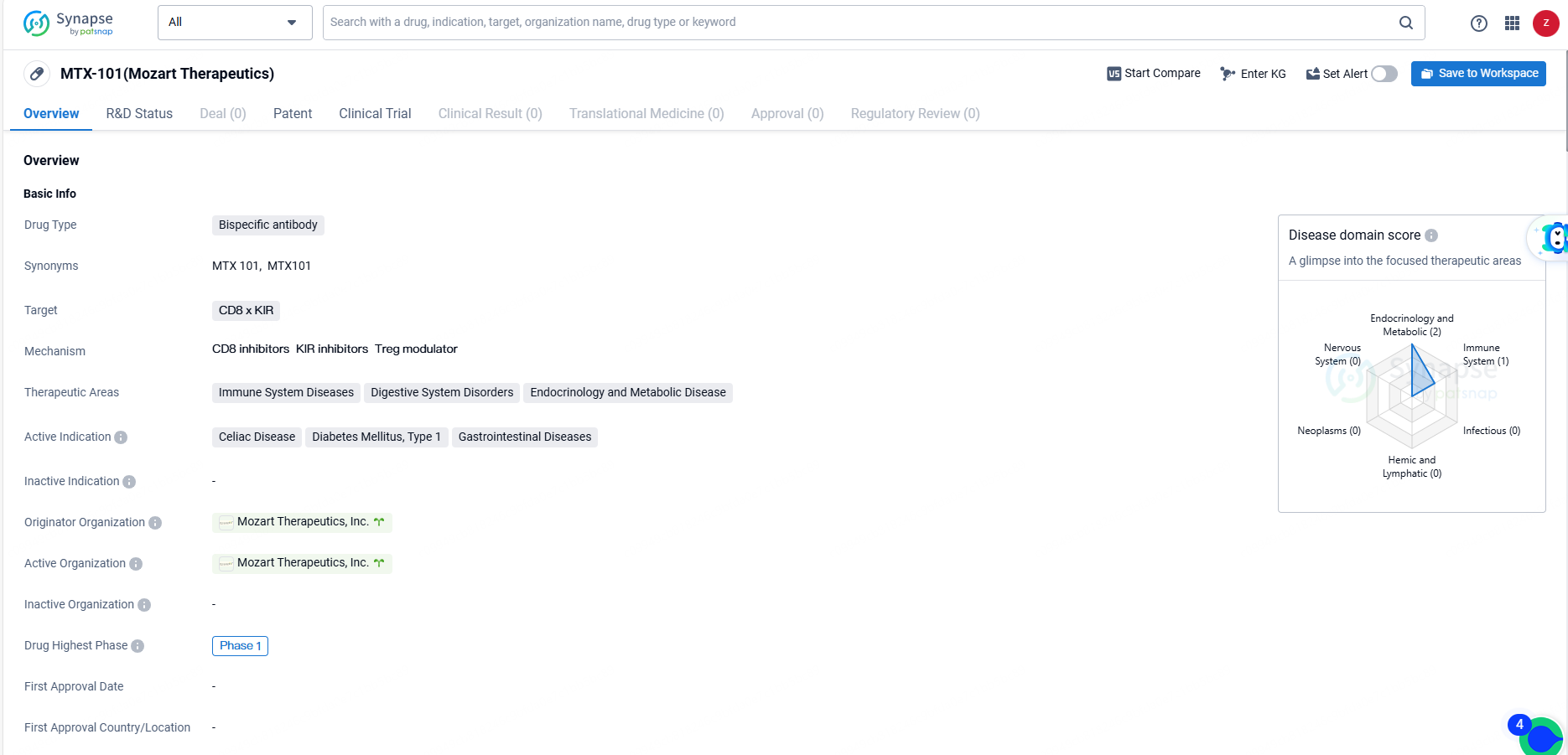
The Phase 1a segment of the trial involves single and multiple ascending dose escalation studies that assess the safety, pharmacokinetics, and pharmacodynamics of MTX-101 in healthy volunteers. Phase 1b consists of a forward-looking, multi-site, randomized, double-blind, placebo-controlled study aimed at evaluating the safety, PK, PD, and disease-specific biomarkers in individuals with type 1 diabetes mellitus or celiac disease.
“The purpose of this trial is to meticulously profile a new method for reestablishing immune balance, while also acknowledging the pressing need for safer, more efficacious targeted strategies to prevent and manage chronic, debilitating autoimmune conditions. Upon completing Phase 1a, we anticipate generating proof-of-mechanism data in two distinct and clinically different autoimmune diseases, and ideally demonstrate the widespread applicability of CD8 Treg biology,” noted Dr. Jason Chien, Chief Medical Officer at Mozart.
MTX-101 is a bispecific antibody targeting the inhibitory KIR and CD8 molecules found on regulatory CD8 T cells. This autoimmune checkpoint inhibitor aims to restore the inherent functions of regulatory CD8 T cells, acting at the early stages of autoimmune disease to suppress and eliminate pathogenic cells, reduce downstream inflammation, and prevent tissue damage.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
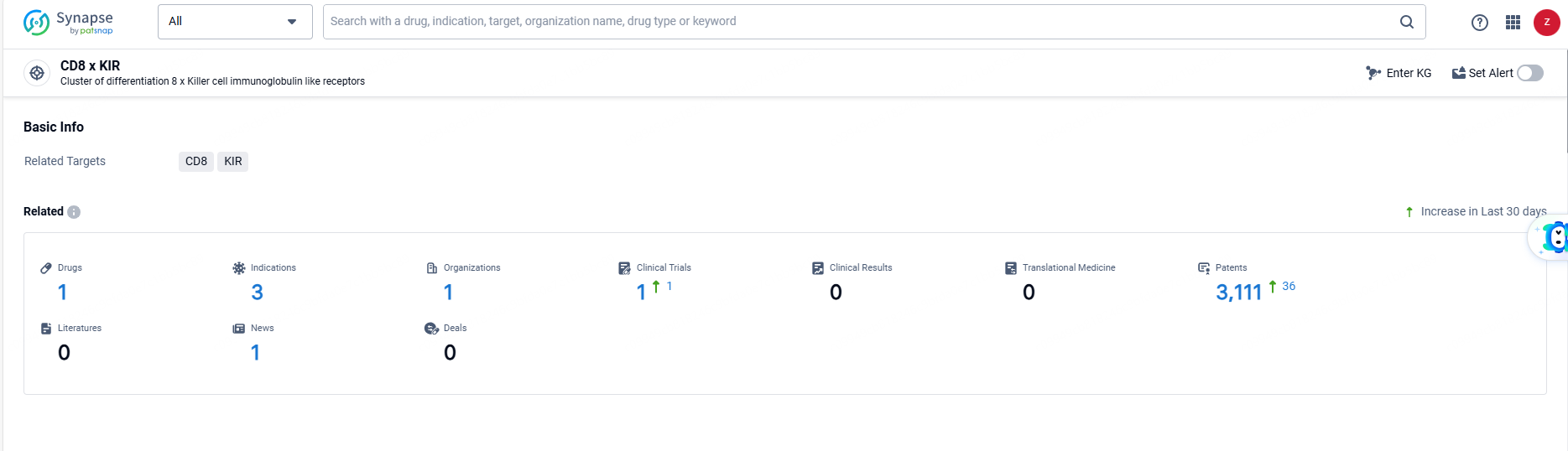
According to the data provided by the Synapse Database, As of June 21, 2024, there are 1 investigational drugs for the CD8 and KIR target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 3111 patents.
MTX-101 is a bispecific antibody drug targeting CD8 x KIR, with a focus on immune system diseases, digestive system disorders, and endocrinology and metabolic diseases. The drug is currently in Phase 1 of clinical development, and its active indications include celiac disease, diabetes mellitus type 1, and gastrointestinal diseases. As the development progresses, further clinical data and regulatory milestones will be important to monitor the potential of MTX-101 as a therapeutic option in the pharmaceutical industry.