Is Recarbrio approved by the FDA?
Yes, Recarbrio is FDA approved. The U.S. Food and Drug Administration (FDA) approved Recarbrio, which is a combination of imipenem, cilastatin, and relebactam, on July 16, 2019.
What is Recarbrio?
Recarbrio is a combination antibiotic consisting of imipenem, cilastatin, and relebactam. This combination belongs to the drug class of carbapenems and beta-lactamase inhibitors. It is formulated as an intravenous powder for injection, with each dose containing 1.25 grams of the active ingredients.
Uses of Recarbrio
Recarbrio is used to treat various complicated infections in adults who have limited or no alternative treatment options. These infections include:
- Complicated Urinary Tract Infections (cUTI):
- Includes pyelonephritis, a type of kidney infection.
- Caused by bacteria such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
- Complicated Intra-abdominal Infections (cIAI):
- Infections within the stomach area.
- Caused by bacteria such as Bacteroides fragilis, Escherichia coli, and Klebsiella pneumoniae.
- Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP):
- Lung infections acquired in a hospital setting or from the use of ventilators.
- Caused by bacteria such as Klebsiella pneumoniae, Pseudomonas aeruginosa, and Serratia marcescens.
Dosage
Recarbrio is administered as an intravenous infusion with the typical dosage being 1.25 grams every six hours. The duration of treatment generally ranges from 4 to 14 days depending on the severity and type of infection.
Side Effects
Common Side Effects:
- Increased blood pressure
- Nausea, diarrhea, vomiting
- Abnormal liver function tests
- Fever
- Headache
- Injection site reactions (pain, bruising, swelling, irritation)
Serious Side Effects:
- Tremors
- Seizures
- Severe stomach pain or diarrhea (watery or bloody)
Warnings and Precautions
- Allergic Reactions: Patients allergic to imipenem, cilastatin, or relebactam should not use Recarbrio.
- Seizures: Use with caution in patients with a history of seizures.
- Antibiotic-associated Diarrhea: Diarrhea may indicate a new infection; consult a doctor if severe diarrhea occurs.
Drug Interactions
Recarbrio may interact with other medications such as divalproex sodium, ganciclovir, and valproic acid. Patients should inform their healthcare provider of all medications they are taking to avoid potential interactions.
Conclusion
Recarbrio, approved by the FDA in July 2019, is a vital antibiotic used to treat severe and complicated infections in patients with few or no other treatment options. Its approval underscores its significance in combating resistant bacterial infections, making it a crucial option in the treatment of complicated urinary tract infections, intra-abdominal infections, and certain types of pneumonia.
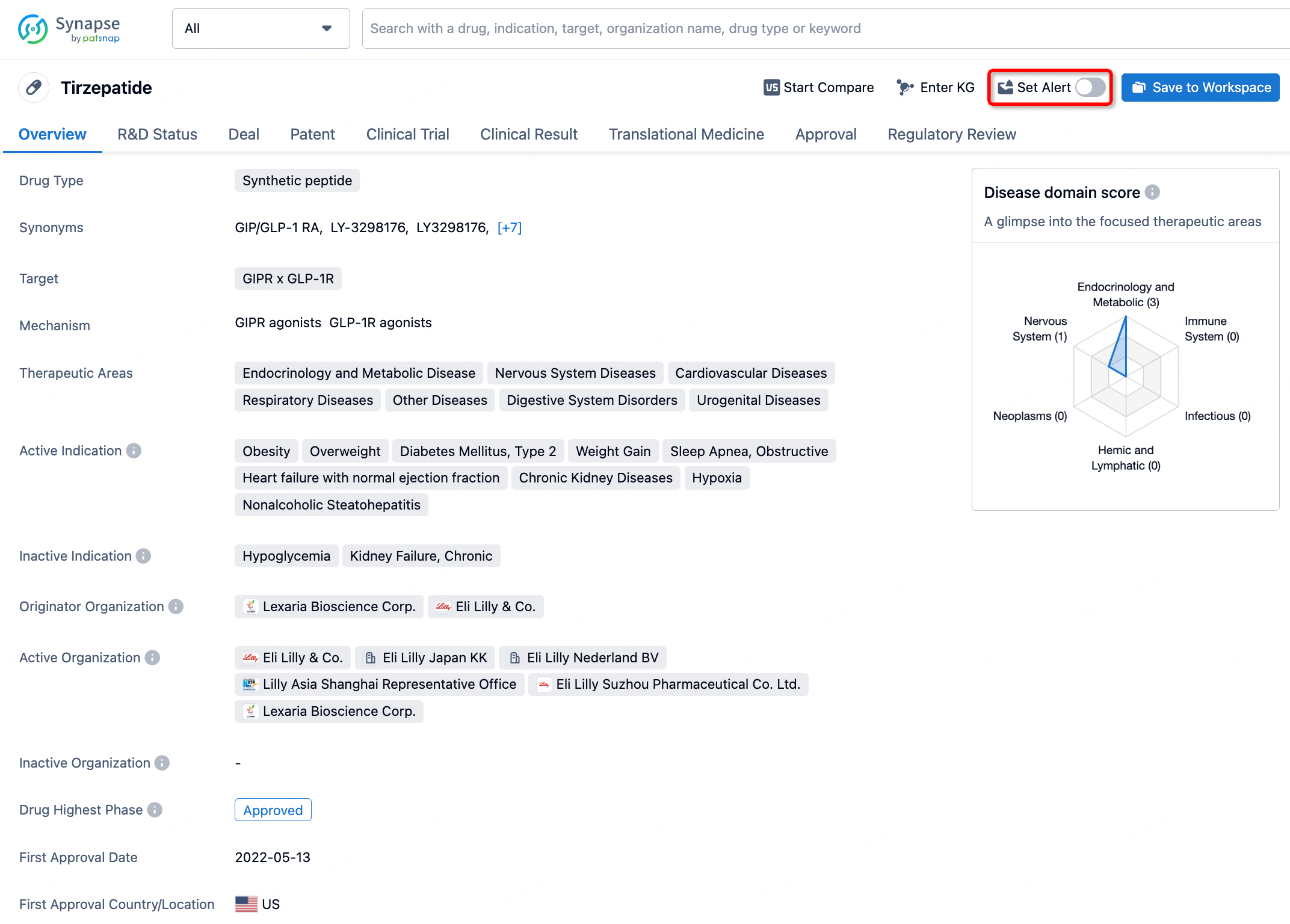
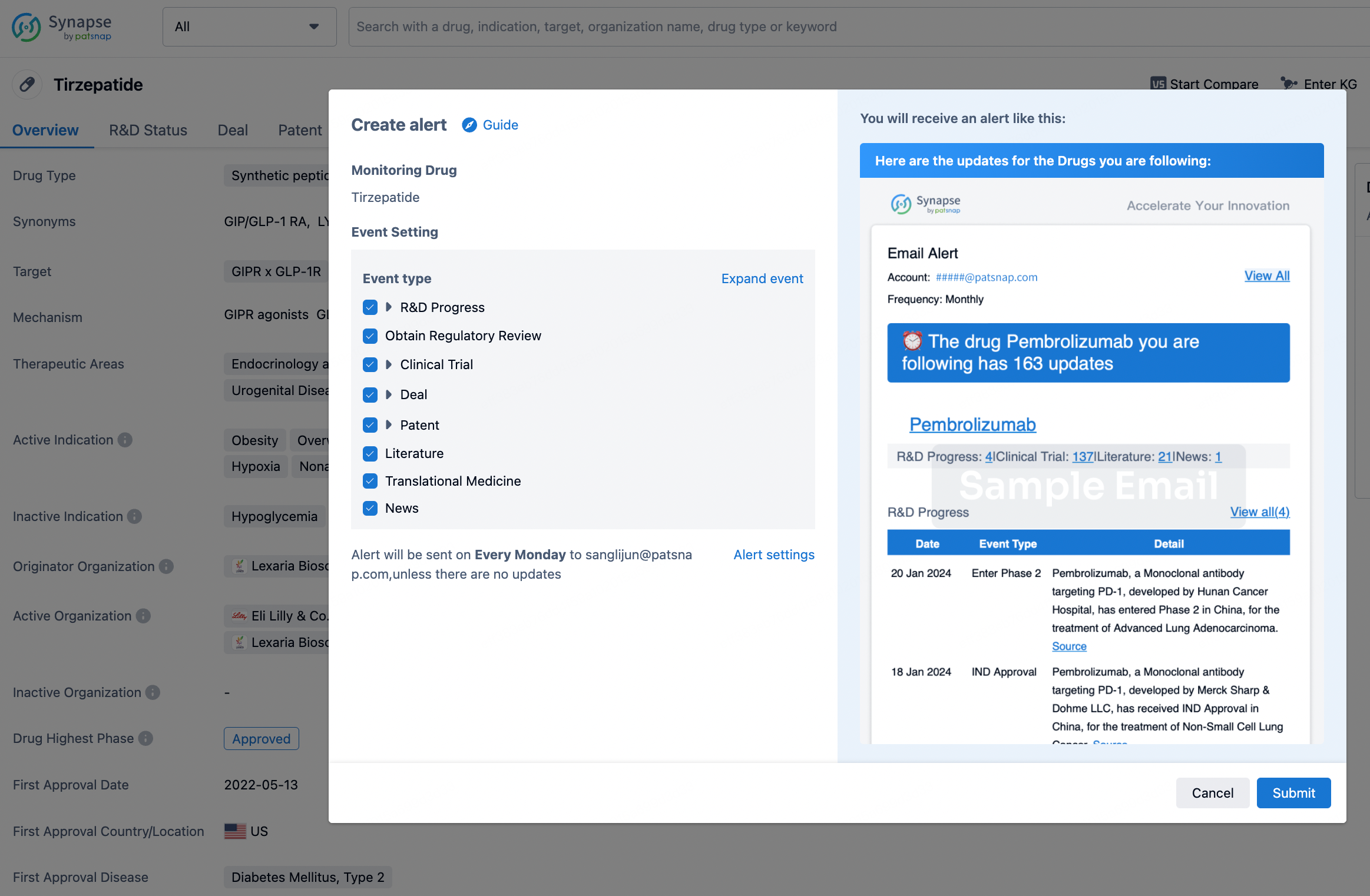
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!