mRNA Vaccines: Embracing Change and Moving Forward
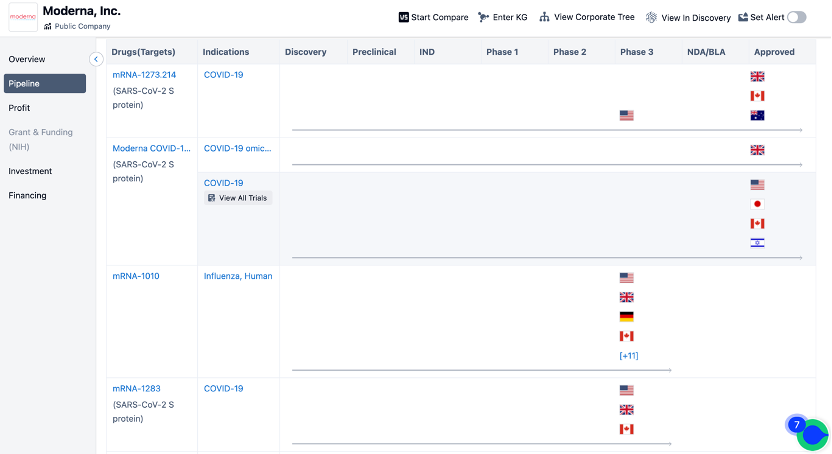
In April 2023, at the American Association for Cancer Research (AACR) annual meeting, Moderna and Merck announced detailed information about the phase 2b clinical trial of mRNA-4157 in combination with Keytruda for the treatment of melanoma.
The study demonstrated that in patients with advanced melanoma, approximately 79% of patients who received Moderna's mRNA-based cancer vaccine in combination with Merck's immune checkpoint inhibitor Keytruda remained recurrence-free after 18 months, compared to 62% of patients who received Keytruda alone. Both companies stated their plans to initiate phase 3 clinical trials by the end of this year.
Following the success of mRNA vaccines against COVID-19, tumor treatment is poised to become the next significant field for mRNA vaccines. We have entered a new era in cancer therapy, although there is still some way to go before widespread product application.
mRNA vaccines involve delivering mRNA encoding antigen proteins into the human body, which is then translated directly into antigens, inducing an immune response. Unlike DNA and viral vector-based vaccines, mRNA vaccines are non-integrating and non-infectious, eliminating the potential risk of infection or insertional mutations and offering a higher level of safety. Additionally, mRNA has transient expression in the body, allowing for repeated administration.
The outbreak of the COVID-19 pandemic has facilitated the successful application of mRNA vaccines, but beyond infectious diseases, these products have tremendous potential in the field of tumor treatment.
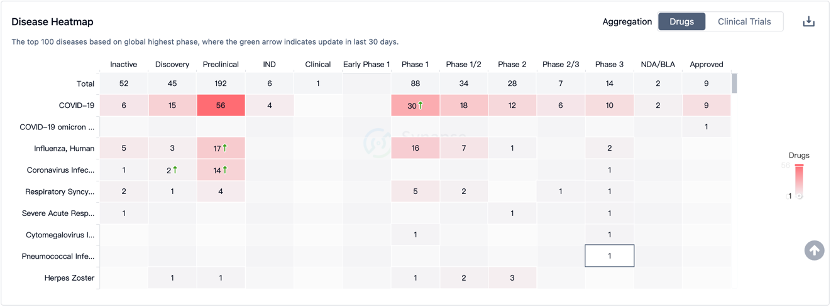
According to Boston Consulting Group's forecast, the entire mRNA vaccine market is projected to reach $23 billion by 2035. Within this market, mRNA cancer vaccines are expected to account for a 32% share, approximately $7.36 billion. This field of research is rapidly advancing at an unprecedented pace and showing promising prospects.
Compared to many infectious disease vaccines, only a few cancer vaccines have been approved for human use. Cancer vaccines refer to those that can stimulate the body to generate active, specific immune responses against cells or molecules favorable for tumor growth, either within tumor cells or the tumor microenvironment (TME).
Cancer vaccines can be classified into preventive and therapeutic categories. Currently, well-known vaccines such as HPV and HIV belong to the preventive category, while therapeutic cancer vaccines have yet to be commercialized on a global scale.
In the form of mRNA vaccines, specific tumor antigen proteins can be synthesized within the human body, thereby inducing a targeted immune response against tumors. Unlike existing PD-1 inhibitors and CAR-T cell therapies, mRNA cancer vaccines can mobilize the entire immune system of patients, triggering a more potent and specific immune response.
Tumor antigens can be categorized into two types: tumor-associated antigens (TAA) and tumor-specific antigens (TSA). TAAs are expressed in both normal tissues and tumors, but they are typically overexpressed in tumors.
As a result, TAAs have weaker tumor specificity and immunogenicity and are susceptible to central immune tolerance. This has been one of the main challenges in developing cancer vaccines. TSAs, also known as neoantigens, differ from TAAs in that they are typically not expressed in normal cells, possess stronger tumor specificity and immunogenicity, and can bypass central T-cell tolerance to self-epitopes.
TSAs are observed in most tumors. A study analyzing HLA-bound peptides across 13 different tumors (a total of 2,488 samples) revealed that each tumor may generate dozens to thousands of different neoantigens.
However, it is important to note that TSAs exhibit high heterogeneity, with the types and quantities of mutations varying even within the same patient. This poses challenges in the screening and identification of TSAs, which are considered optimal targets for anti-tumor therapies.
While mRNA synthesis is fast and cost-effective (GMP-grade mRNA can be produced within three weeks), the prediction phase for neoantigens may require more time and resources. Furthermore, during the vaccine preparation process, patients' conditions may change, potentially leading to missed optimal treatment windows.
However, fortunate enough, the accuracy of neoantigen prediction and identification has been improved through the application of current large-scale high-throughput sequencing methods and artificial intelligence.
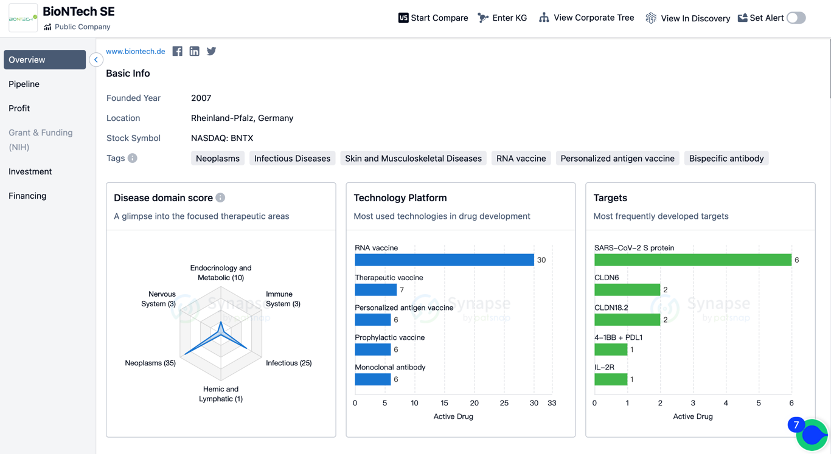
The emphasis on these two different types of antigens roughly represents the two directions in current development of universal and personalized tumor vaccines. However, this categorization is not absolute. Upon reviewing the pipelines of vaccine giants like BioNTech and Moderna, it is evident that both companies have ventured into these areas.
(Click on the image below to access the detailed information on BioNTech/Moderna's research institutions, including investigational drugs, indications, clinical trials, and more.)
During the development of tumors, central immune tolerance and peripheral immune tolerance to tumor-associated antigens (TAAs), such as immune checkpoint pathways and the tumor microenvironment, pose two major challenges that limit the effectiveness and duration of cancer vaccines.
To address central immune tolerance, combinations of multiple TAAs or multiple tumor-specific antigens (TSAs) can be used. To overcome peripheral immune tolerance, the concurrent administration of immune checkpoint inhibitors (such as anti-PD-(L)1 and anti-CTLA-4) and other drugs can be employed.
Furthermore, regarding the antigen prediction challenge faced by personalized mRNA cancer vaccines, as individual data accumulates, it is gradually becoming possible to establish shared neoantigen libraries to address antigen prediction issues for the same or different types of cancer. Although this is a lengthy process, it is achievable. Over time, this will significantly accelerate the production process.
Currently, there is a consensus in the industry regarding the application prospects of mRNA cancer vaccines, which are primarily focused on inoperable tumors and the prevention of recurrence after surgery, radiation therapy, or chemotherapy. Researchers are integrating multiple approaches to advance mRNA cancer vaccines at the forefront of immunotherapy to achieve better therapeutic outcomes.
For example, the combination of Moderna's mRNA cancer vaccine mRNA-4157 and Merck's PD-1 therapy Keytruda has shown promising results in Phase II clinical trials, marking a breakthrough in the clinical validation of mRNA cancer vaccine concepts. However, it is important to acknowledge that there is still a considerable journey ahead before the actual products can be implemented.