Neurizon's NUZ-001 Decreases TDP-43 Aggregation, a Key Target in ALS, in Preclinical Research
Neurizon Therapeutics Limited (ASX: NUZ & NUZOA), a biotech company focused on clinical developments for neurodegenerative conditions, is excited to report favorable outcomes from a preclinical evaluation of its primary candidate, NUZ-001. These groundbreaking studies demonstrate NUZ-001’s distinct mechanism that inhibits the aggregation of TAR DNA-binding protein 43 (TDP-43), which is a critical pathological characteristic of ALS. Additionally, NUZ-001 has shown a substantial improvement in the electrophysiological dysfunction of TDP-43 M337V mutated motor neurons, highlighting its potential as a revolutionary treatment for ALS. These results are particularly significant as they support the encouraging efficacy data obtained in Neurizon’s Phase 1 MEND trial, further enhancing confidence in the therapeutic potential of NUZ-001 for individuals diagnosed with ALS.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Two distinct preclinical investigations were carried out in partnership with Ncardia, a prominent firm specializing in human induced pluripotent stem cell (iPSC) technology. The initial study focused on assessing the efficacy of NUZ-001 along with its primary active metabolite, NUZ-001 Sulfone, in mitigating TDP-43 aggregation within M337V Motor Neurons that were co-cultured with astrocytes under stressful conditions. TAR DNA-binding protein 43 (TDP-43) is recognized as a significant factor in the pathology of ALS. Findings indicated that both NUZ-001 and NUZ-001 Sulfone significantly diminished TDP-43 aggregation in M337V Motor Neurons exposed to the aggregation stressor MG-132, achieving reductions of approximately 50% and 55%, respectively, in a dose-dependent manner.
The follow-up study assessed the capability of NUZ-001 and NUZ-001 Sulfone to restore normal electrophysiological activities in TDP-43 mutated M337V Motor Neurons. This specific mutation has been linked to ALS pathology and is known to disrupt neuronal electrical activity across various dimensions. Both NUZ-001 and NUZ-001 Sulfone enhanced the electrical activity in TDP-43 M337V Motor Neurons by increasing bursting and network burst activities, while simultaneously decreasing inter-burst intervals to levels comparable to wild type Motor Neurons.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
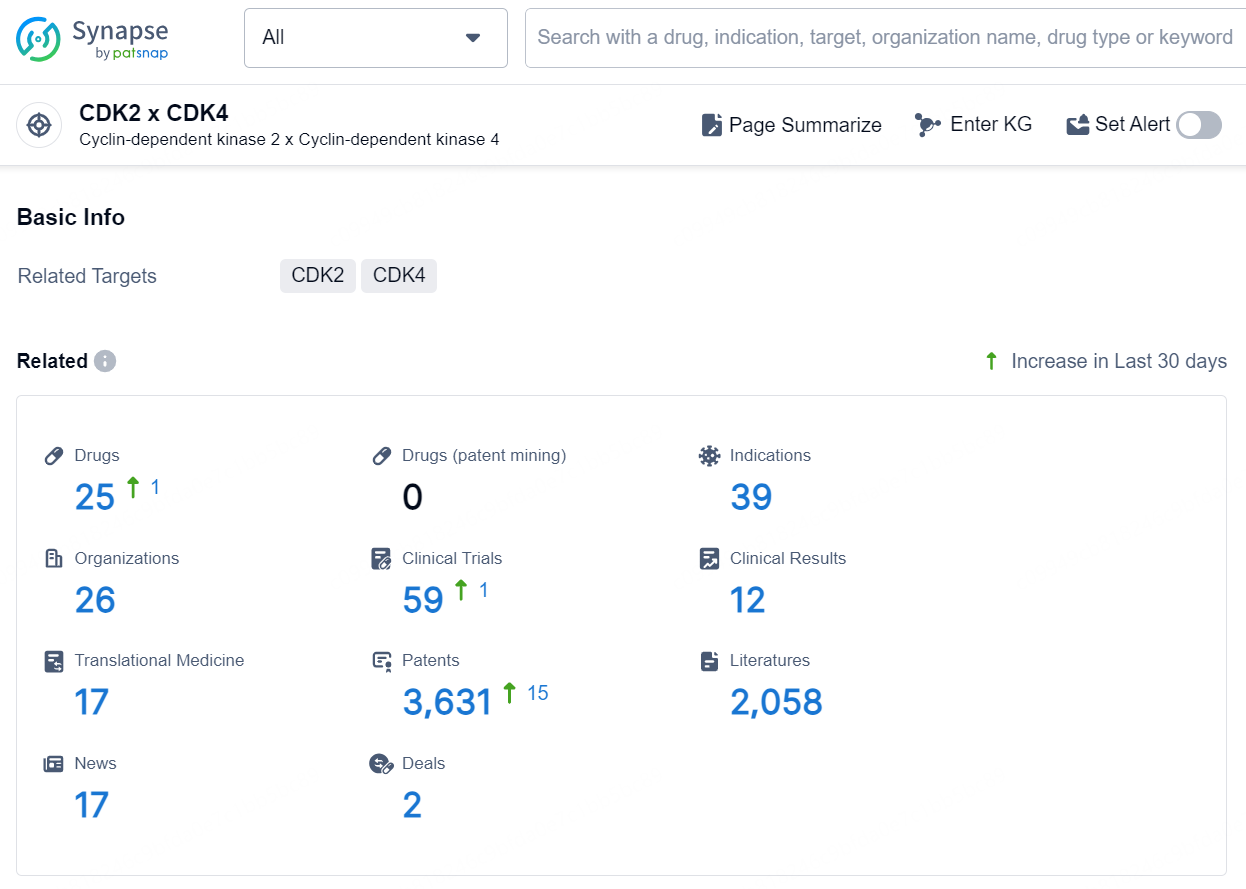
According to the data provided by the Synapse Database, As of November 25, 2024, there are 25 investigational drugs for the CDK2 x CDK4 target, including 39 indications, 26 R&D institutions involved, with related clinical trials reaching 59, and as many as 3631 patents.
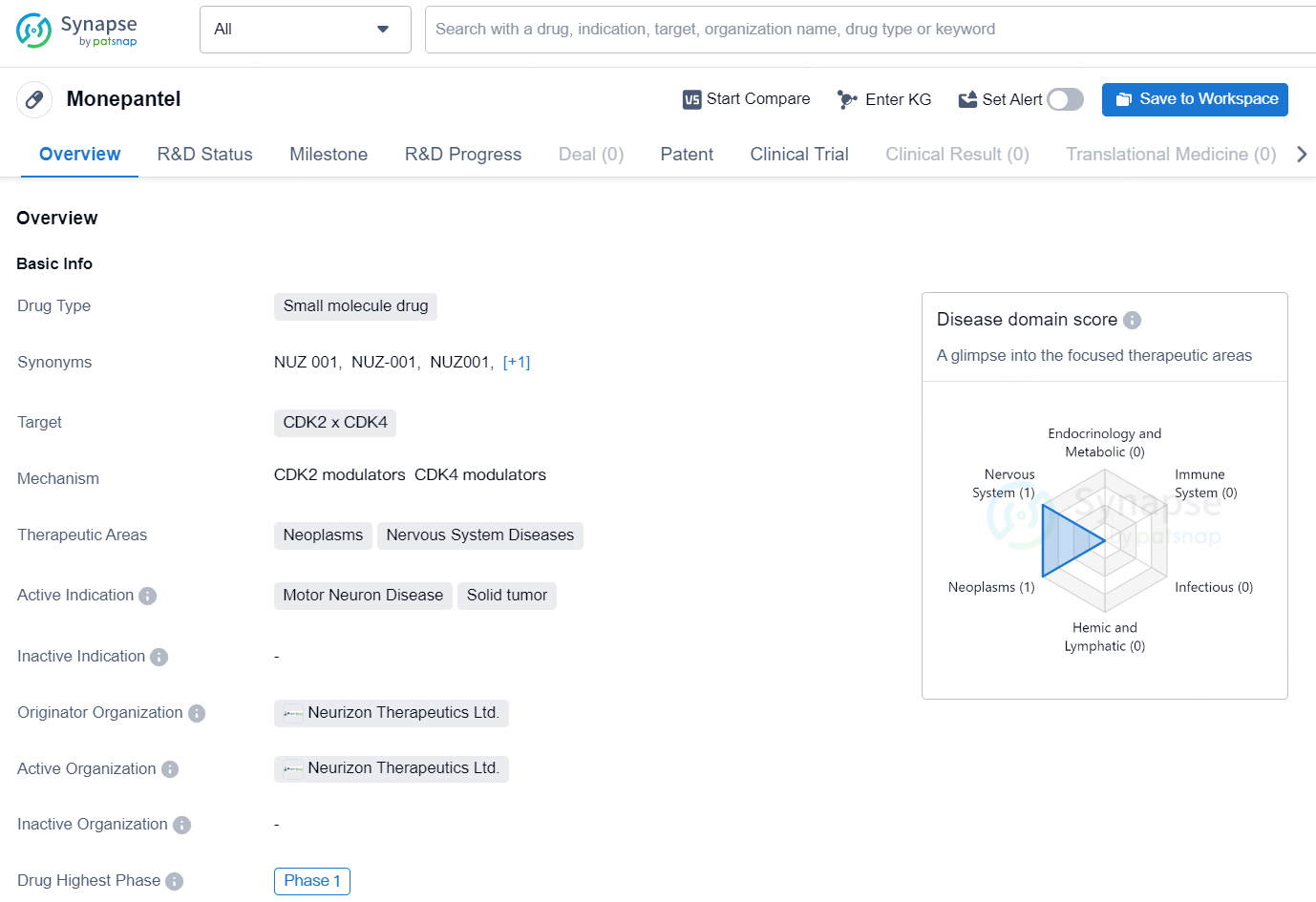
Monepantel is a small molecule drug that targets CDK2 and CDK4, making it suitable for therapeutic areas such as neoplasms and nervous system diseases. The active indications for Monepantel include motor neuron disease and solid tumors. The drug is developed and patented by Neurizon Therapeutics Ltd., and it has reached Phase 1 in its development.