NexThera Submits IND for Early-Stage Clinical Trial of NT-101 Eye Drops to Treat Wet AMD
NexThera Co., Ltd. announced the submission of a Phase 1/2a Investigational New Drug application to the U.S. Food and Drug Administration for NT-101, a non-invasive eye drop therapy aimed at treating wet age-related macular degeneration.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
NT-101 represents NexThera's inaugural new drug candidate produced via its unique eye drop delivery platform. This cutting-edge technology facilitates the non-invasive and safe delivery of compounds to the retinal tissue, eliminating the necessity for direct eye injections.
The formulation of NT-101 includes an endogenous peptide as its active pharmaceutical ingredient alongside a carrier protein. The agent has demonstrated either comparable or superior therapeutic effects relative to current antibody-based therapies. The carrier protein significantly enhances retention on the ocular surface and optimizes drug delivery to the retinal tissue, offering greater efficacy than the active ingredient alone.
Unlike traditional treatments that mainly focus on VEGF inhibition and may cause adverse effects, NT-101 provides dual advantages by not only inhibiting angiogenesis but also protecting optic nerve cells. This is achieved through a balanced modulation of angiogenic and anti-angiogenic factors.
According to a NexThera representative, “The phase 1/2a clinical trial for NT-101 aims to assess the safety and tolerability of this investigational drug in patients with wet AMD. The trial will involve administering low or high doses of the drug to 30 patients, twice daily for a period of 28 days. Secondary endpoints include tracking changes in central subfield thickness and best-corrected visual acuity post-administration.”
CEO SaeGwang Park of NexThera expressed confidence, saying, “We believe that the NT-101 clinical trial will establish this drug as a pioneering treatment for wet AMD and set the stage for its further development. We are also investigating its potential use in other retinal neovascular conditions, such as diabetic retinopathy.”
Founded in February 2020, NexThera is a biopharmaceutical research and development entity utilizing platform technology. The company has grown through strategic collaborations and investments, including joint development agreements with EyeGene Inc. and BMI Korea Co., Ltd. NexThera has successfully raised a total of KRW 16.3 billion through multiple funding rounds, including seed funding, Series A investment, and bridge round investment.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
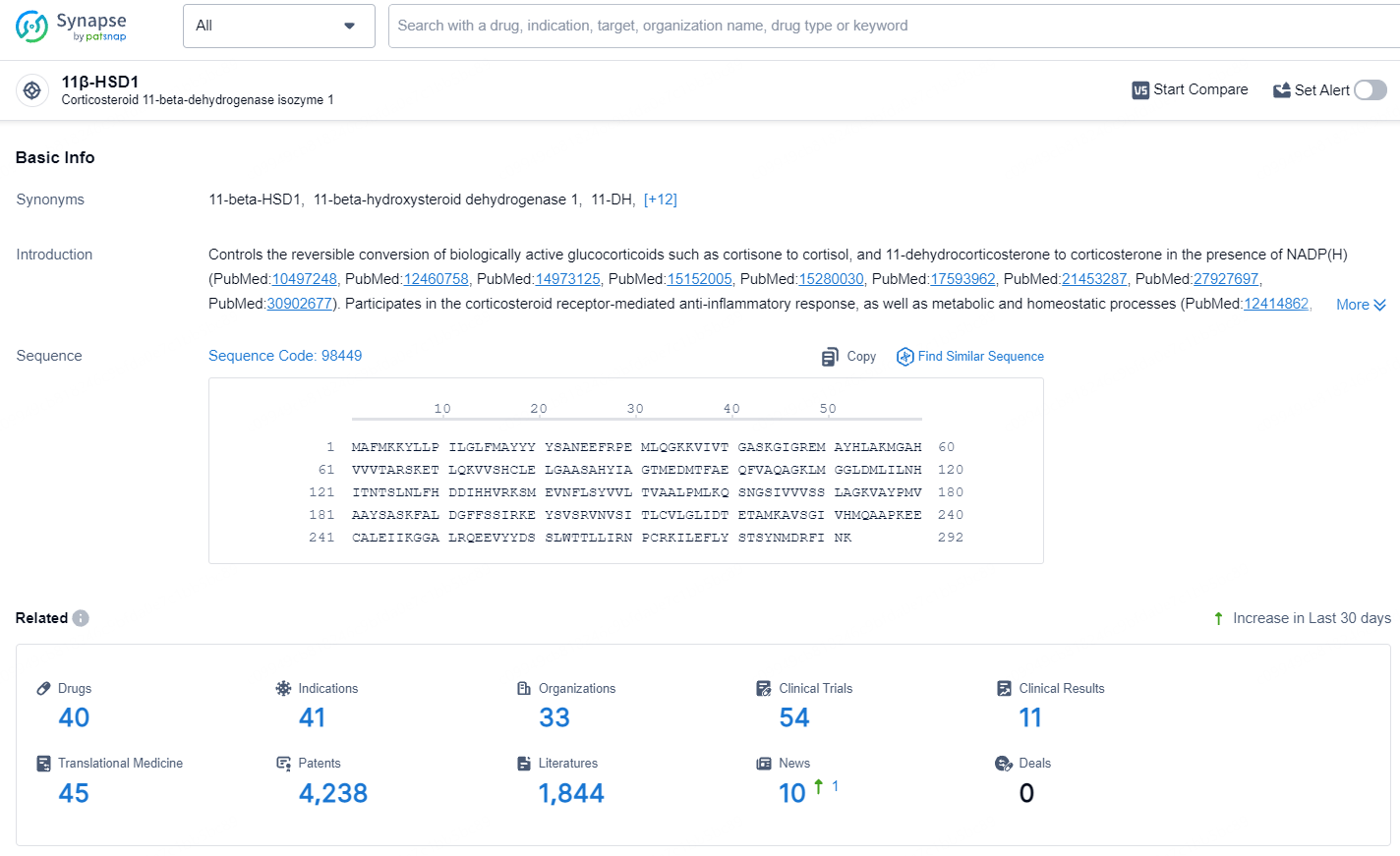
According to the data provided by the Synapse Database, As of July 22, 2024, there are 40 investigational drugs for the 11β-HSD1 target, including 41 indications, 33 R&D institutions involved, with related clinical trials reaching 54, and as many as 4238 patents.
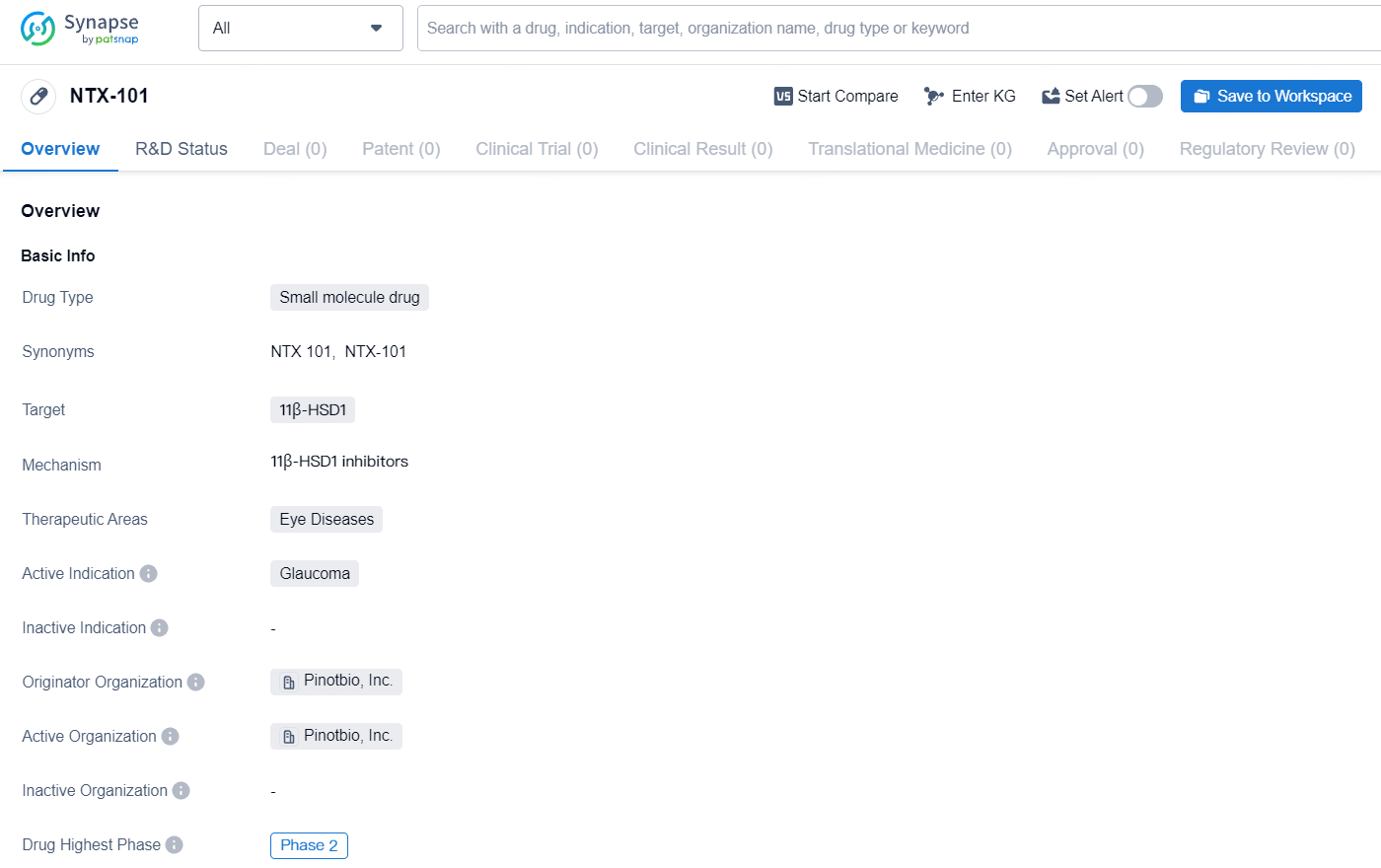
NTX-101 is a small molecule drug developed by Pinotbio, Inc. that targets the 11β-HSD1 enzyme. The therapeutic focus of NTX-101 is on treating eye diseases, with a specific active indication for glaucoma. As of the latest available information, NTX-101 has progressed to Phase 2 of clinical development, making it an advanced candidate in the drug development process.The choice of a small molecule drug for targeting 11β-HSD1 suggests a potential for oral administration, which may offer advantages in terms of patient convenience and adherence. The specific targeting of 11β-HSD1 indicates a focus on modulating cortisol levels, which may have implications for the treatment of glaucoma and other related eye diseases.