NGM Bio Raises $122M in Series A for New Clinical Trials on Primary Sclerosing Cholangitis and Hyperemesis Gravidarum
NGM Biopharmaceuticals, Inc., a biotechnology enterprise privately funded and dedicated to the discovery and development of breakthrough therapeutics for patients, has declared a Series A financing totaling $122 million. This funding round was spearheaded by TCG with participation from a select group of investors. The capital raised will be allocated by NGM Bio to commence a registrational trial of aldafermin, a specially engineered FGF19 analog, aimed at treating PSC, as well as to finalize a Phase 2 trial of NGM120, a GDF15/GFRAL antagonist, which is intended for the treatment of HG. Both clinical studies are slated to start in the last quarter of 2024.
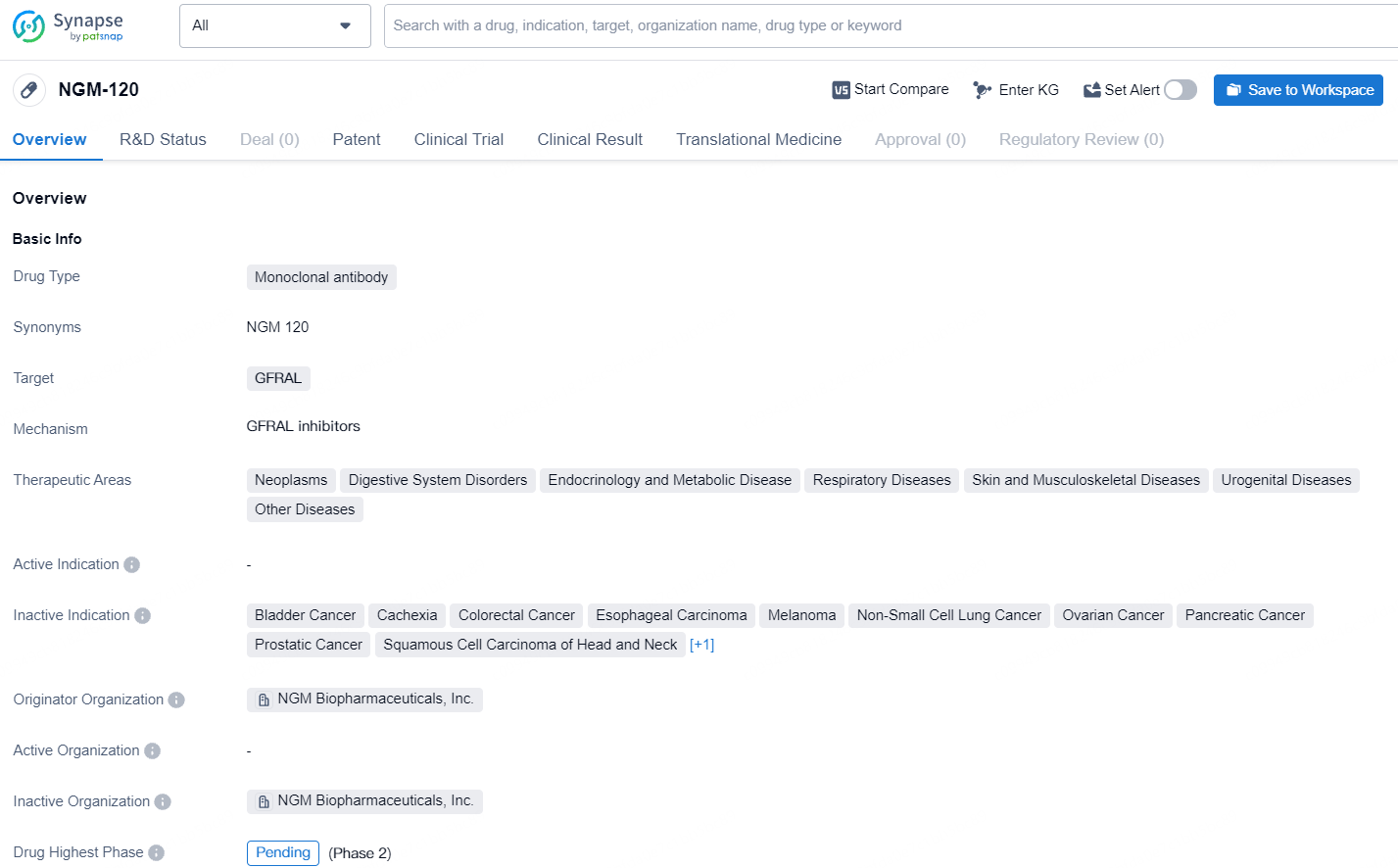
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"At the outset of this year, we revealed our plan to promote clinical advancement for two chronic, rare conditions marked by a substantial unmet need for patients. This Series A funding bolsters our financial status, allowing us to move forward with our planned registrational trial of aldafermin for primary sclerosing cholangitis and to conduct a proof-of-concept study of NGM120 for hyperemesis gravidarum,” stated David J. Woodhouse, PhD, Chief Executive Officer of NGM Bio.
"The Column Group has consistently supported NGM’s goal to transform exceptional scientific findings into impactful treatments. NGM has demonstrated impressive tenacity and dedication in its quest for life-saving therapies while adhering to the highest standards of scientific excellence. With this Series A funding, TCG is pleased to deepen our investment in NGM and to back the promising opportunities offered by aldafermin and NGM120 as potential treatments for PSC and HG, respectively,” expressed Peter Svennilson, Founder and Managing Partner of TCG. Alongside the Series A financing, Mr. Svennilson joined NGM Bio’s Board of Directors.
PSC is a rare illness that severely damages the bile ducts, causing bile acid mishandling, potentially leading to end-stage liver disease, liver failure, and bile duct cancer. NGM Bio’s progression of aldafermin as a prospective treatment for PSC is founded on substantial clinical data that supports its unique potential to address the bile acid dysregulation underlying the disease, including NGM Bio’s earlier Phase 2 study in PSC. NGM Bio expects that the registrational trial for aldafermin in PSC will use proposed surrogate endpoints with the objective of obtaining accelerated approval.
HG is marked by severe nausea and vomiting, which lead to dehydration, physical weakness, weight loss, and poor nutrition. HG significantly impacts physical and mental health and is associated with higher rates of fetal loss, preeclampsia, preterm birth, low birth weight, fetal malnutrition, maternal depression, and in some cases, suicidal thoughts. It is the second leading cause of hospitalization during pregnancy (following preterm labor) and often recurs in subsequent pregnancies.
The development of NGM120 as a potential treatment for HG stems from NGM Bio’s extensive efforts over the past decade to advance understanding of GDF15 biology and explore its therapeutic applications in various diseases. Recent genetic studies confirm the connection between HG and elevated serum levels of GDF15. NGM120 is an antibody aimed at inhibiting GDF15 signaling through its specific receptor, GFRAL, located in cells at the brain base that are involved in vomiting and nausea, potentially providing therapeutic benefits for HG patients."
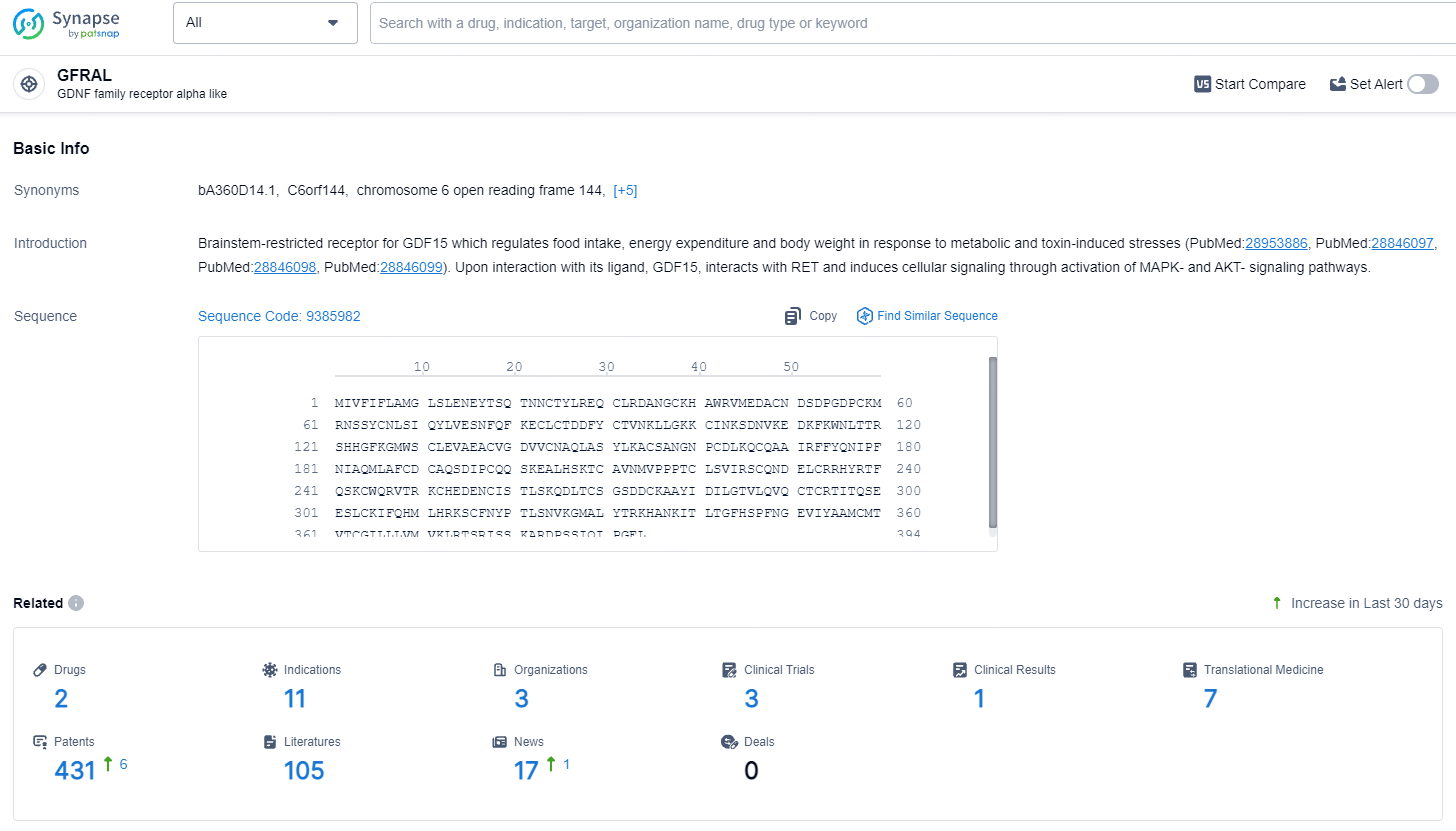
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 23, 2024, there are 2 investigational drugs for the GFRAL targets, including 11 indications, 3 R&D institutions involved, with related clinical trials reaching 3, and as many as 431 patents.
NGM-120 as a monoclonal antibody targeting GFRAL represents a significant advancement in biomedicine with potential implications for the treatment of diverse medical conditions. As the drug progresses through the development pipeline, it will be important to continue monitoring its developments and future milestones.