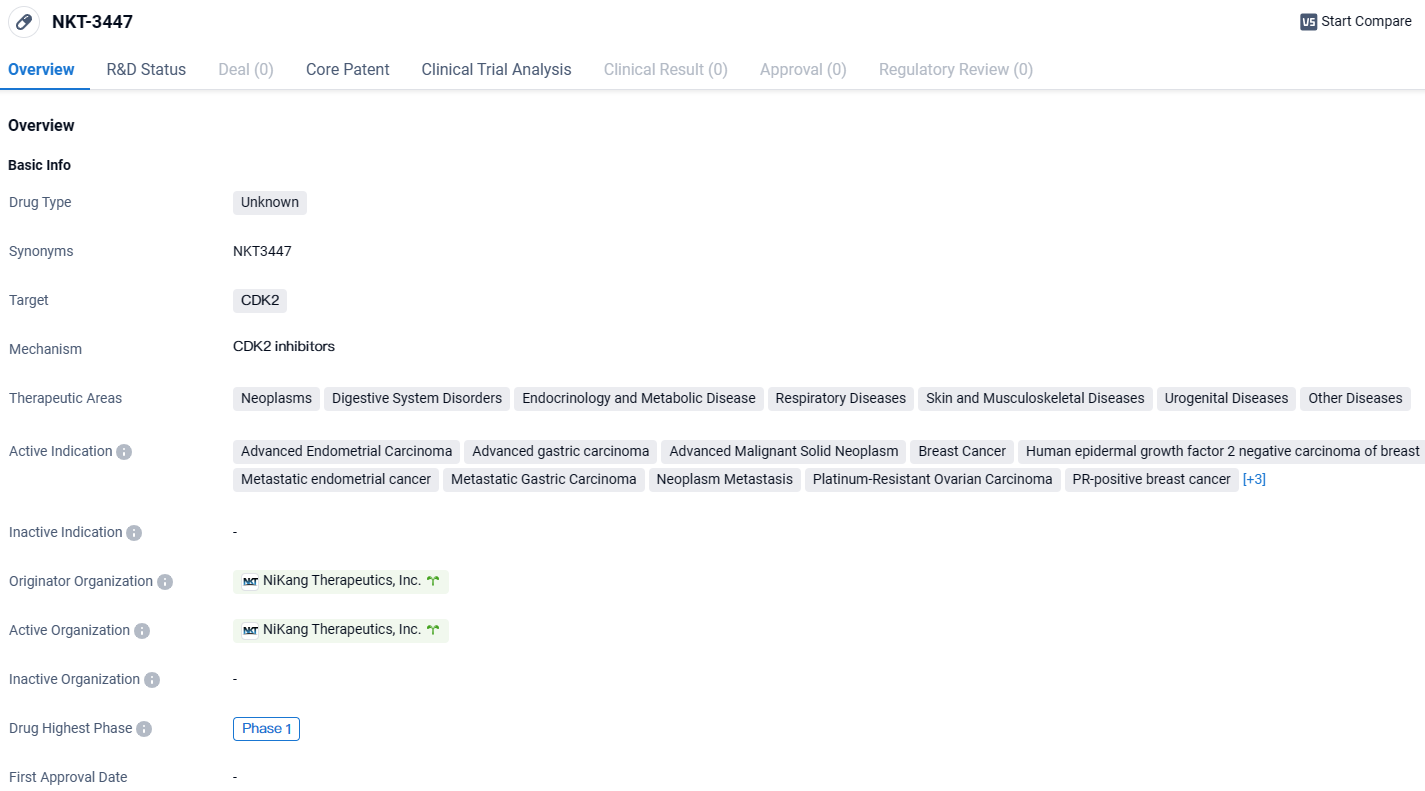
NiKang Therapeutics Begins Phase 1/1b Trial with First Dose of Oral CDK2 Inhibitor NKT3447
NiKang Therapeutics Inc., a biotechnology firm in the clinical phase, is dedicated to crafting novel small molecule drugs for oncology to address the unmet needs of patients. The firm recently declared that it has commenced dosing the initial participant in a phase 1/1b open-label study. This first-in-human trial focuses on dose escalation and expansion using the single agent NKT3447, a small molecule designed to block cyclin-dependent kinase 2 (CDK2).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
NKT3447 has been developed specifically for treating patients whose cancers are characterized by either an amplification or overexpression of cyclin E, a common occurrence across multiple tumor varieties. The preliminary Phase 1/1b clinical trial aims to assess various critical factors such as safety, tolerability, pharmacokinetic and pharmacodynamic profiles, and the overall clinical efficacy of NKT3447 in adult patients with advanced or metastatic solid tumors, associated with aberrations in cyclin E or CDK2.
Marking a significant progress, NiKang has initiated the dosing in these trials, as stated by Zhenhai Gao, Ph.D., the co-founder, president, and CEO of NiKang. He noted, “The commencement of this trial with NKT3447 represents an important development, being the foremost among our projects focusing on the cell cycle to reach the clinical trial phase.”
Dr. Gao further elaborated, “Our belief in CDK2 as a crucial target in oncology is strong, and we’ve developed a comprehensive portfolio which includes novel compounds like a CDK2-selective degrader and a dual degrader impacting CDK2/4. Even though drugs targeting the cell cycle have achieved clinical milestones, pinpointing CDK2 inhibitors that do not adversely interact with CDK1 or trigger an unwanted increase in cyclin E — a key factor in cancer cell growth — remains a significant challenge.”
Adding to the discussion, Joanne Jenkins Lager, M.D., Chief Medical Officer at NiKang, expressed enthusiasm about moving forward with the clinical trials of NKT3447. Dr. Lager highlighted, “NKT3447 distinguishes itself with unique properties leading to prolonged pharmacodynamic impact and pronounced anti-tumor effects in varied models of cyclin E amplified tumors.” She continued, “Given the dysregulation of CDK2 and cyclin E in numerous cancers, we are optimistic about NKT3447’s capability to advance the treatment paradigm for patients with cancers exhibiting cyclin E amplification or overexpression, including those suffering from ovarian, endometrial, and gastric cancers.”
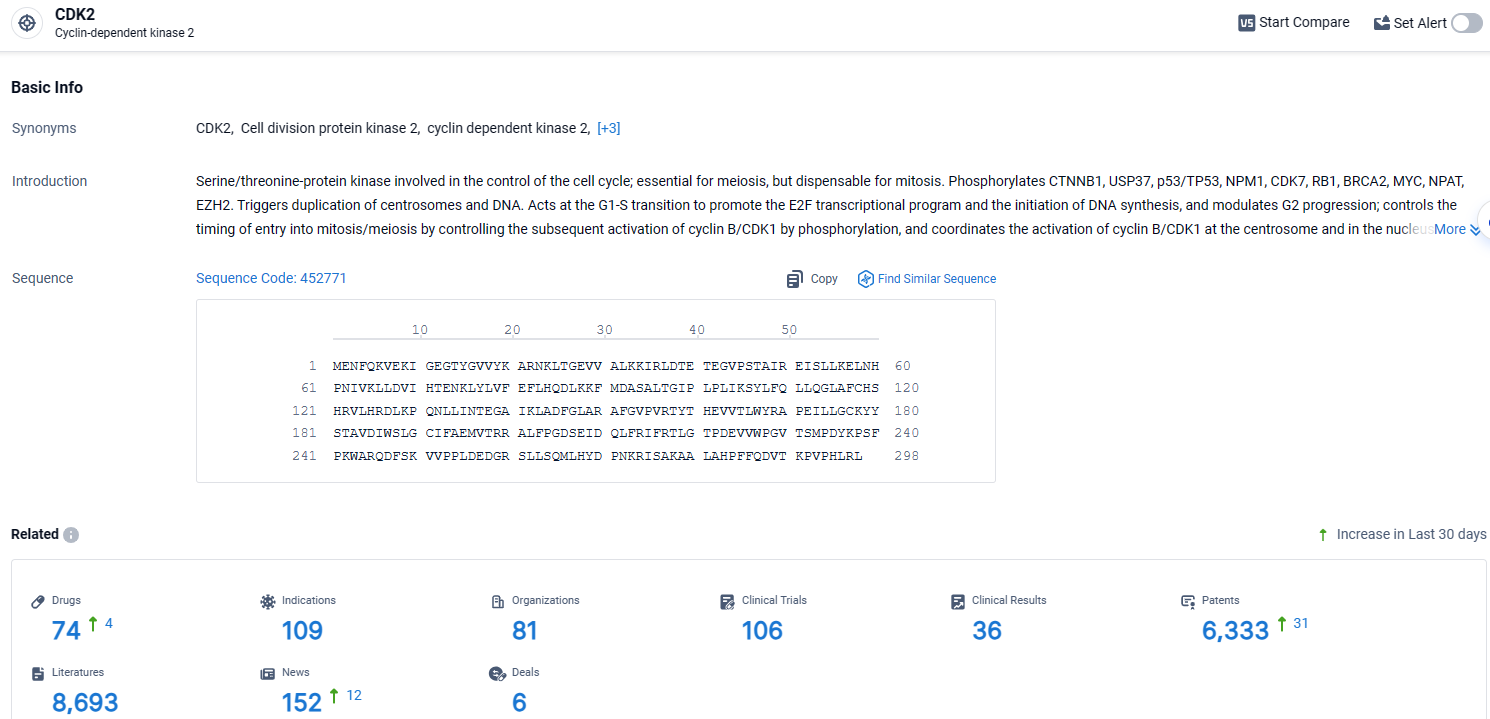
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 17, 2024, there are 74 investigational drugs for the CDK2 target, including 109 indications, 81 R&D institutions involved, with related clinical trials reaching 106, and as many as 6333 patents.
NKT-3447 targets CDK2. It has shown activity in various therapeutic areas, including different types of cancers and other diseases. However, as it is currently in Phase 1, more research and clinical trials are needed to determine its drug type, efficacy, and potential for commercialization.