Decoding CTX-712: a comprehensive study of its R&D trends and its clinical results in 2024 AACR
On April 5, 2024, the first-in-human phase I study of CTX-712 in patients with advanced, relapsed or refractory malignant tumors (CTX-712-CL-01 study) was reported in 2024 AACR.
CTX-712's R&D Progress
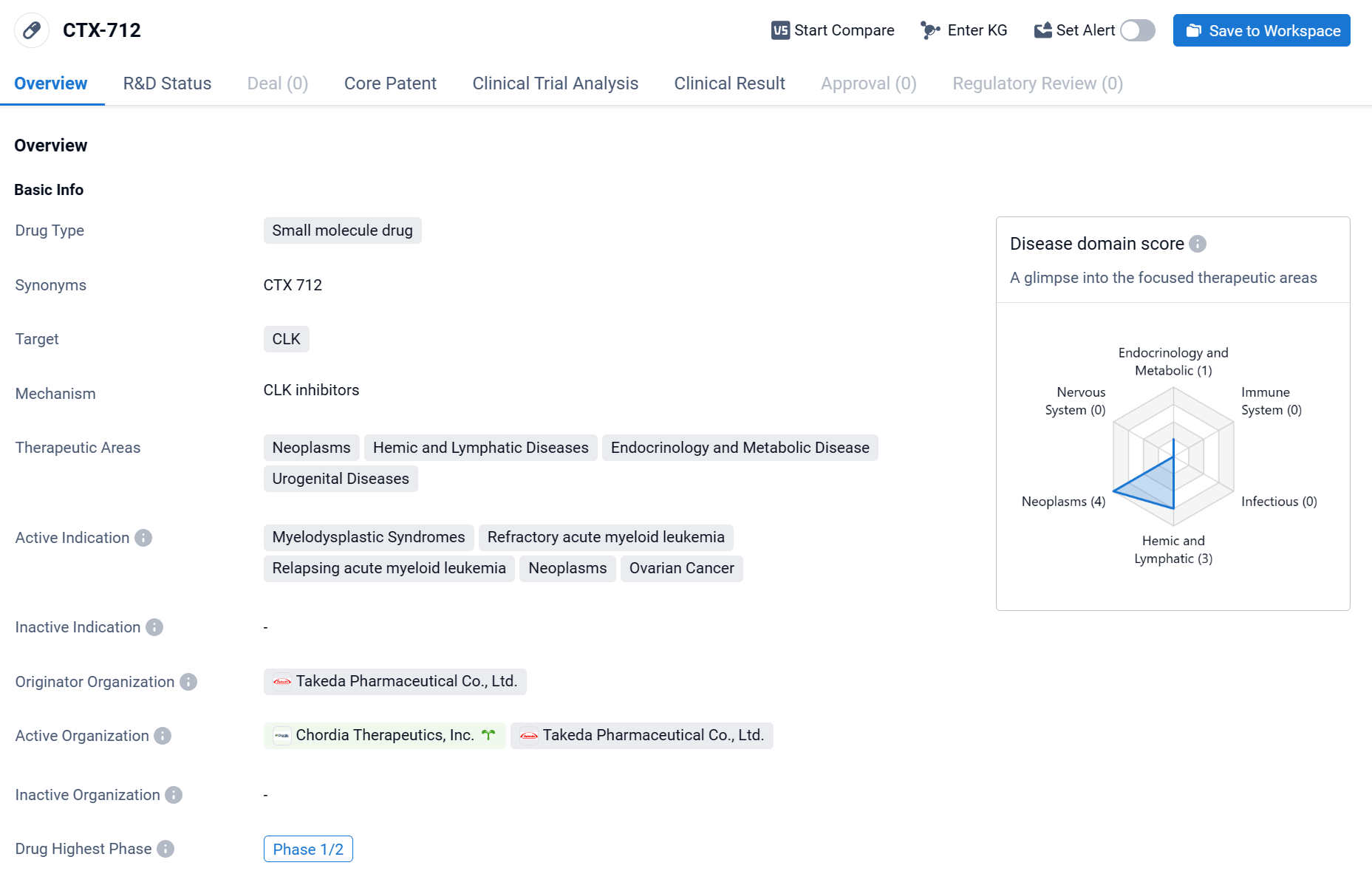
CTX-712 is a small molecule drug that is being developed by Takeda Pharmaceutical Co., Ltd. It is designed to target CLK, a protein involved in various cellular processes. CTX-712 has shown potential in treating a range of therapeutic areas, including neoplasms, hemic and lymphatic diseases, endocrinology and metabolic diseases, and urogenital diseases.
According to the Patsnap Synapse, CTX-712 is currently in the highest phase of clinical development, which is Phase 1/2. And the clinical trial distribution for CTX-712 is primarily in the United States. The key indication is Relapsing acute myeloid leukemia.
Detailed Clinical Result of CTX-712
The objectives of this cohort (JPRN-jRCT2080224127) were to evaluate the dose-limiting toxicity (DLT), safety, pharmacokinetic (PK)/pharmacodynamic (PD) profiles, and preliminary efficacy of CTX-712 in patients with hematologic malignancies.
In this study, a 3+3 design was used in two dose levels (70 mg and 105 mg twice a week (TW)) based on the safety data from the previous dose escalation cohort in patients with solid tumors.
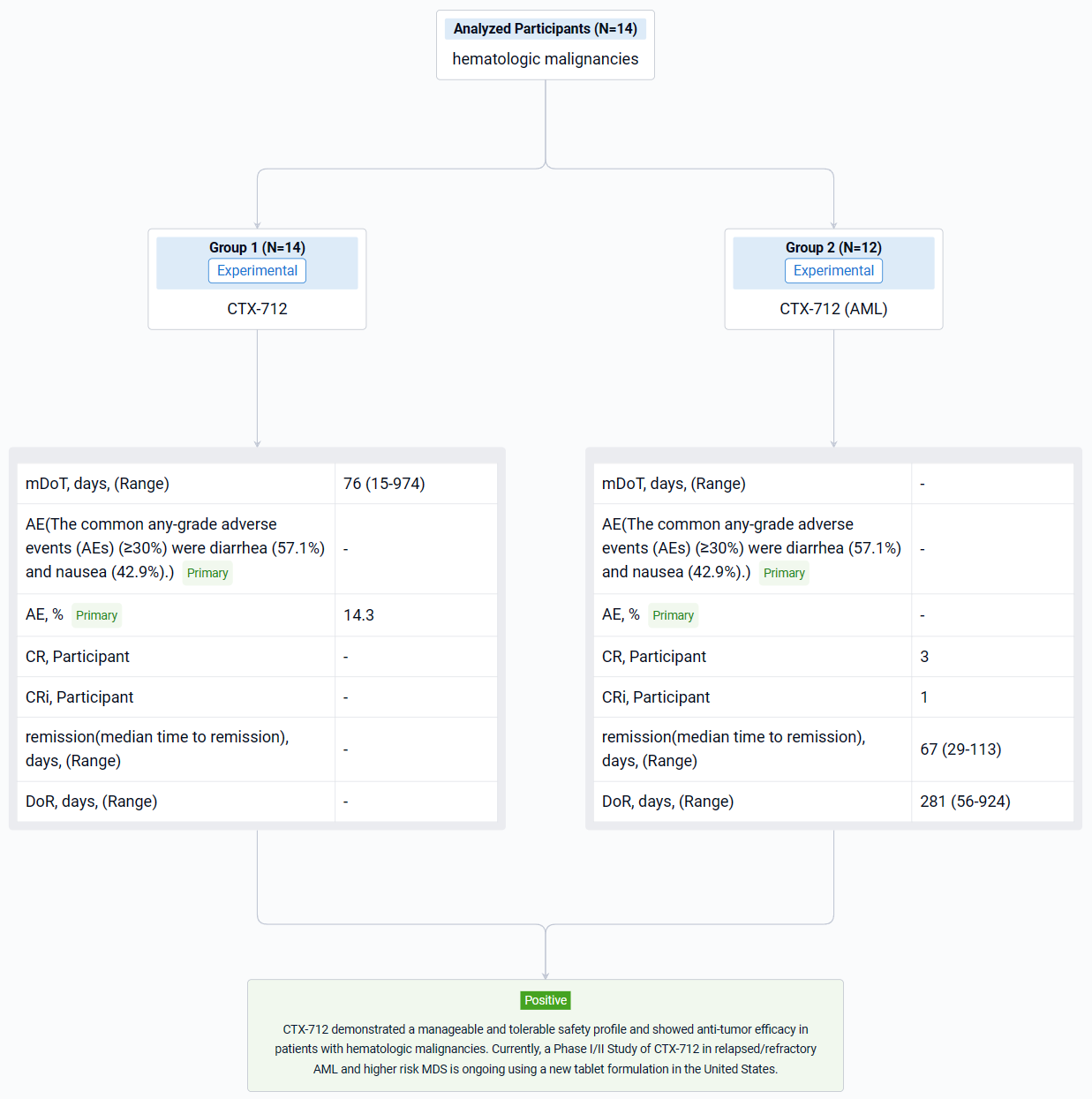
The result showed that as of data cutoff on November 20, 2023, a total of 14 patients (12 AML and 2 MDS) were enrolled in 70 mg TW (n=8) and 105 mg TW dose levels (n=6). DLT of Grade 4 pneumonia was observed in 1 of 3 DLT-evaluable patients in the 105 mg TW group. In the 70 mg TW group, no DLT was observed in 6 DLT-evaluable patients. Among the safety analysis population (n=14), median treatment duration was 76 days (Range 15-974 days). At data cutoff, treatment was ongoing in 1 patient and discontinued in 13 patients. The common any-grade adverse events (AEs) (≥30%) were diarrhea (57.1%) and nausea (42.9%). The most common Grade 3 or higher AE were white blood cell count decreased and tumor lysis syndrome (14.3%). In AML patients (n=12), complete remission (CR) was observed in 3 patients (25.0%), and CR with incomplete hematologic recovery (CRi) was observed in 1 patient (8.3%). The median time to remission was 67 days (Range 29-113 days), and the median duration of response was 281 days (Range 56-924 days). In MDS patients (n=2), CR was observed in 1 patient (50.0%). PK/PD profiles of the hematologic malignancies dose escalation cohort were comparable with those of the solid tumor cohort at the same dosage.
It can be concluded that CTX-712 demonstrated a manageable and tolerable safety profile and showed anti-tumor efficacy in patients with hematologic malignancies. Currently, a Phase I/II Study of CTX-712 in relapsed/refractory AML and higher risk MDS is ongoing using a new tablet formulation in the United States.
How to Easily View the Clinical Results Using Synapse Database?
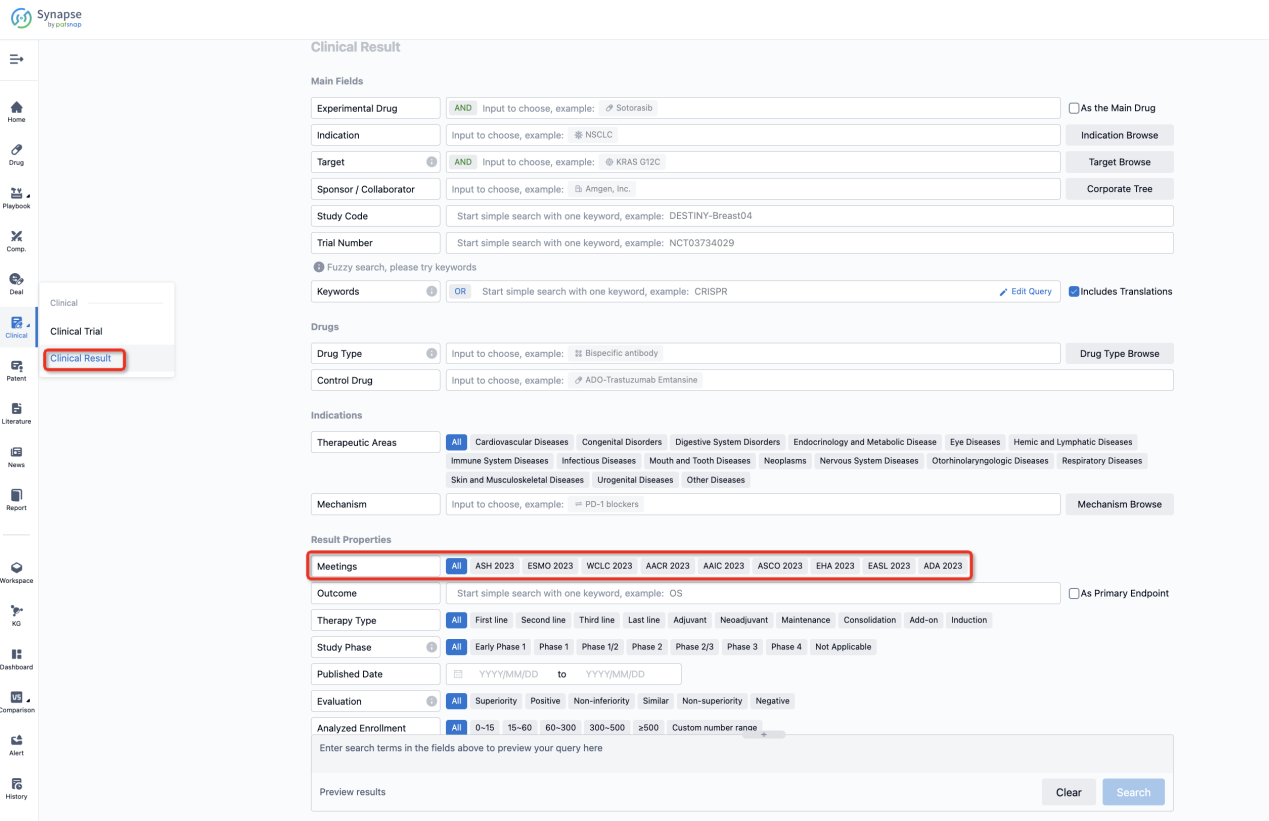
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
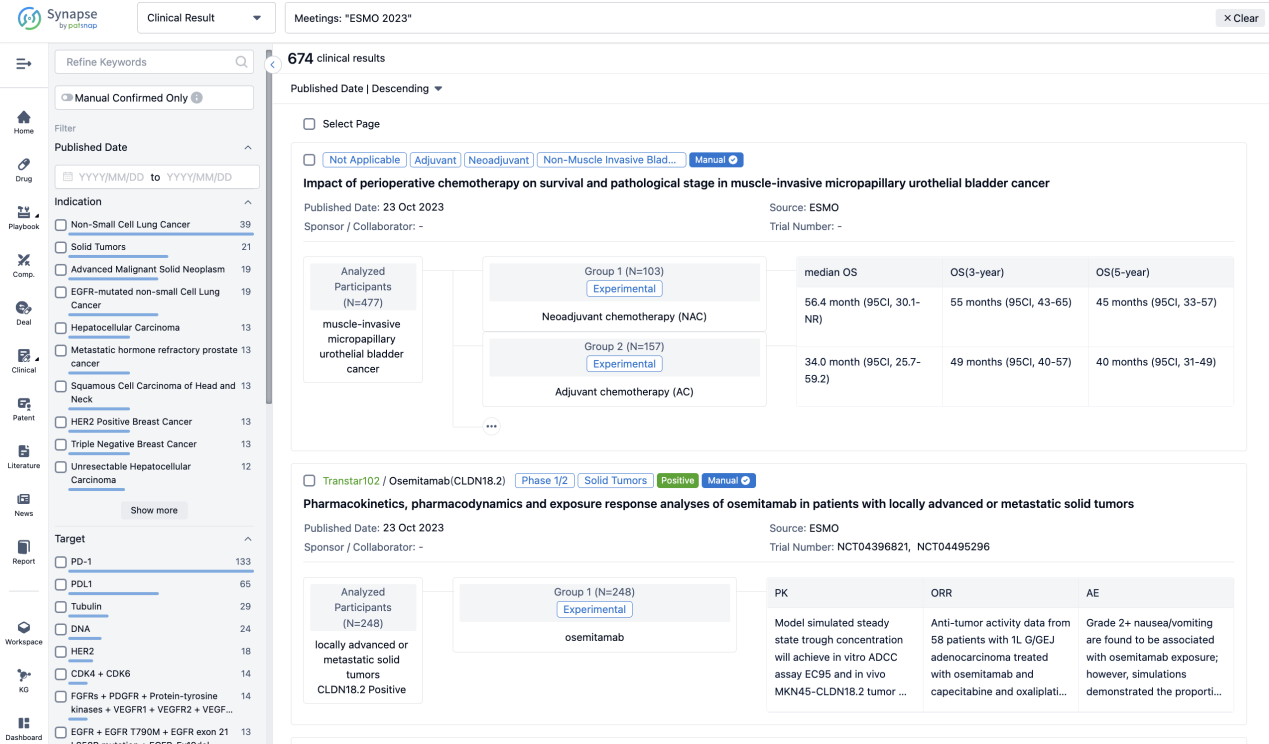
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.


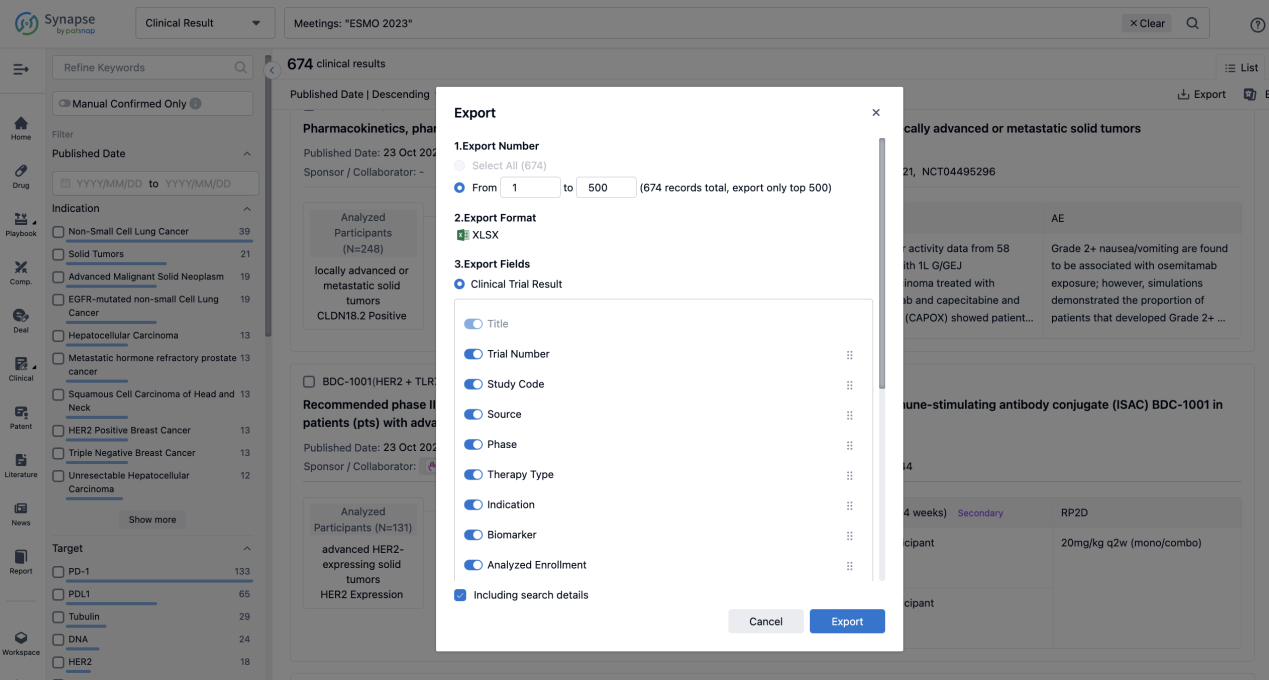
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!