Nivolumab: brief review of its R&D progress and the clinical result in 2023 ASCO
The PD-1 pathway is central to the pathogenesis of HL (Hodgkin lymphoma) and PD-1 blockade is effective in RR (relapsed/refractory) HL. Recently the clinical trial of Nivolumab, a PD-1 blockade, for the treatment of RR HL was reported at the ASCO Congress.
Nivolumab's R&D Progress
Nivolumab is a monoclonal antibody drug that targets PD-1, a protein found on immune cells. It has been approved for use in various therapeutic areas, including neoplasms (tumors), immune system diseases, cardiovascular diseases, endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, digestive system disorders, skin and musculoskeletal diseases, other diseases, nervous system diseases, congenital disorders, and respiratory diseases.
According to the Patsnap Synapse, Nivolumab was first approved in Japan in July 2014 and has since received approvals in other countries. It is developed by Bristol Myers Squibb Co. and Ono Pharmaceutical Co., Ltd. The drug has reached the highest phase of approval globally. And the clinical trial distributions for Nivolumab are primarily in the United States and France. The key indication is Neoplasms.
Detailed Clinical Outcome of Nivolumab
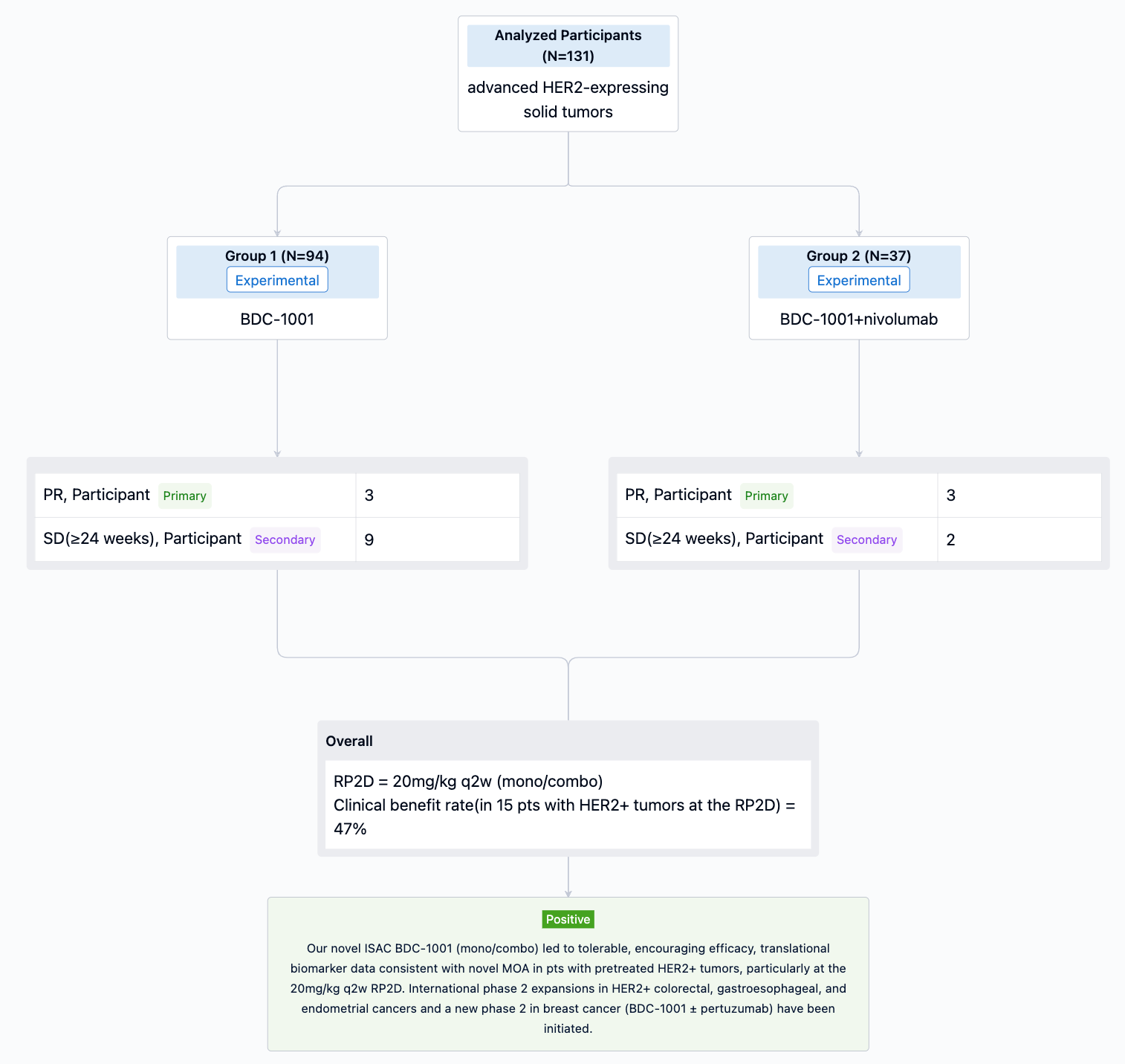
The randomized, parallel assignment, open-labeled clinical trial (NCT03907488) was aimed to evaluate N-AVD vs BV-AVD in pts with newly diagnosed AS HL.
In this study, Eligible pts were ≥12 years (y) with stage 3-4 HL. Pts were randomized 1:1 to either 6 cycles of N-AVD or BV-AVD. Recipients of BV-AVD were required to receive G-CSF neutropenia prophylaxis vs optional with N-AVD. Pre-specified pts could receive RT to residually metabolically active lesions on end of treatment PET. Pts were stratified by age, international prognostic score (IPS), and intent to use RT. Response and disease progression were assessed by investigators using 2014 Lugano Classification. The primary endpoint was PFS; secondary endpoints included OS, event-free survival, patient-reported outcomes (PROs), and safety.
The result showed that 30 PFS events occurred after N-AVD vs 58 events after BV-AVD. With a median follow-up of 12.1 months, PFS was superior in the N-AVD arm [HR 0.48, 99% CI 0.27-0.87, one-sided p=0.0005); 1y PFS: N-AVD, 94%, BV-AVD, 86%. 11 deaths (7 due to adverse events, AE) were observed after BV-AVD compared to 4 after N-AVD (3 due to AE). The rate of grade (gr) ≥ 3 hematologic AE was 48.4% (45.1% gr ≥ 3 neutropenia) after N-AVD compared to 30.5% (23.9% gr ≥ 3 neutropenia) after BV-AVD. Rates (any gr) of febrile neutropenia (5.6% N vs 6.4% BV), pneumonitis (2.0% N vs 3.2% BV), ALT elevation (30.7% N vs 39.8% BV), and colitis (1% N vs 1.3% BV) were similar. Hypo/hyperthyroidism was more frequent after N-AVD (7%/3% N vs <1% BV) while peripheral neuropathy (any gr) was more common after BV-AVD (sensory: 28.1% N vs 54.2% BV; motor: 4% N vs 6.8% BV).
It can be concluded that N-AVD improved PFS vs BV-AVD in pts with AS HL. Few immune AEs were observed and < 1% of pts received RT. Longer follow-up is needed to assess OS and PROs. S1826, the largest HL study in NCTN history, is a key step towards harmonizing the pediatric and adult treatment of AS HL. Funding provided by: National Cancer Institute of the National Institutes of Health U10CA180888 and U10CA180819 and Bristol-Myers Squibb.
How to Easily View the Clinical Results Using Synapse Database?
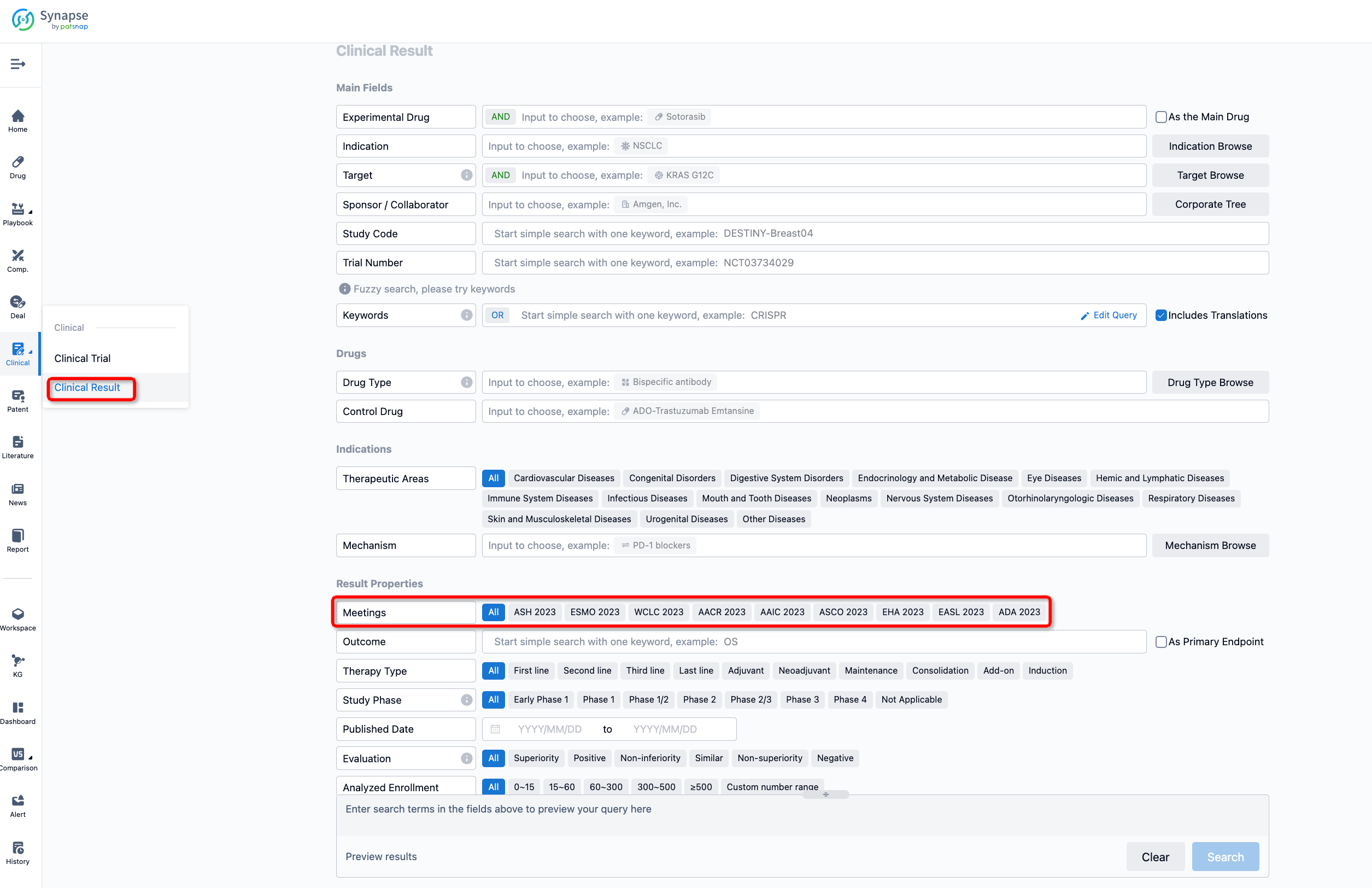
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
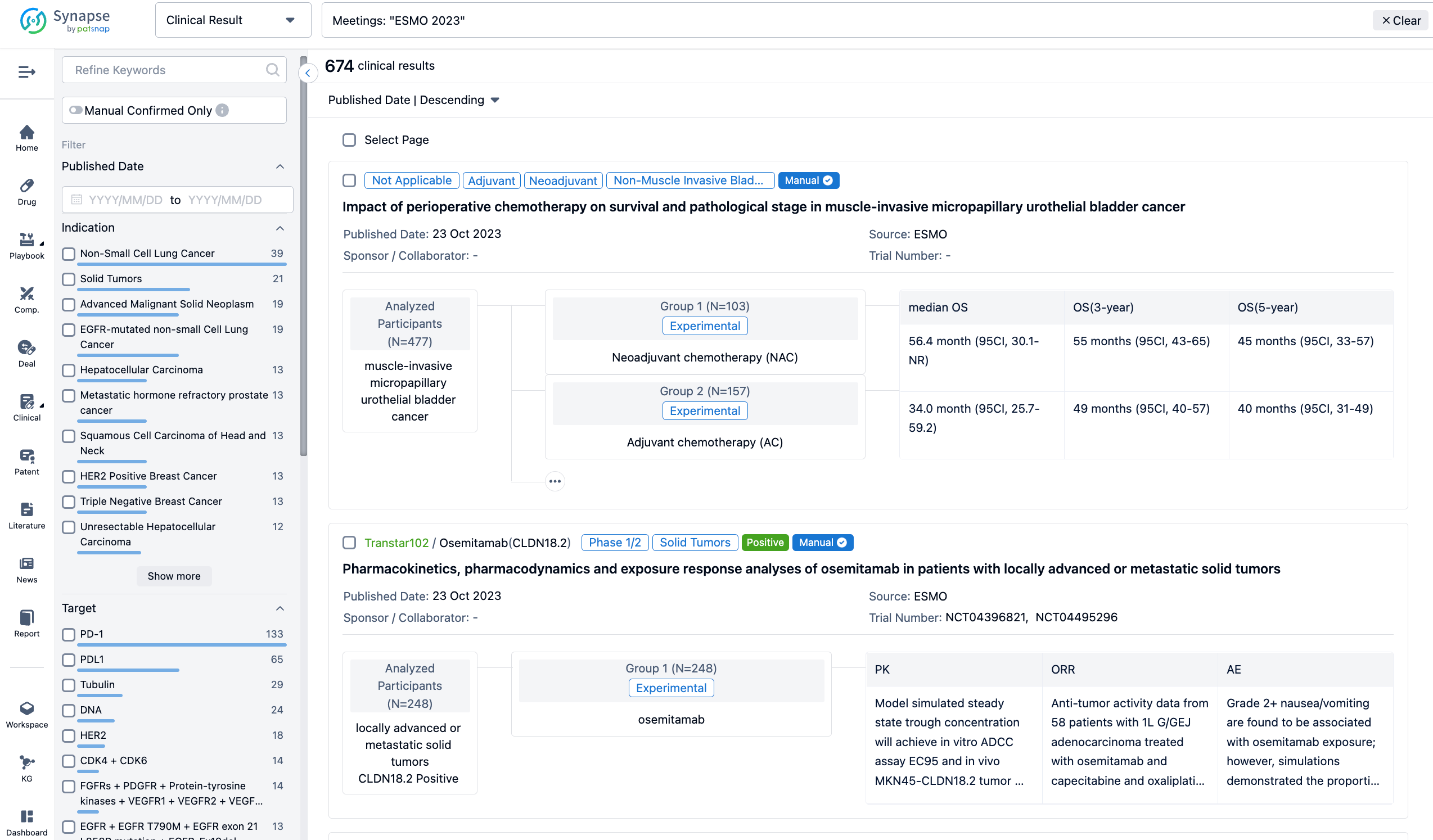
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
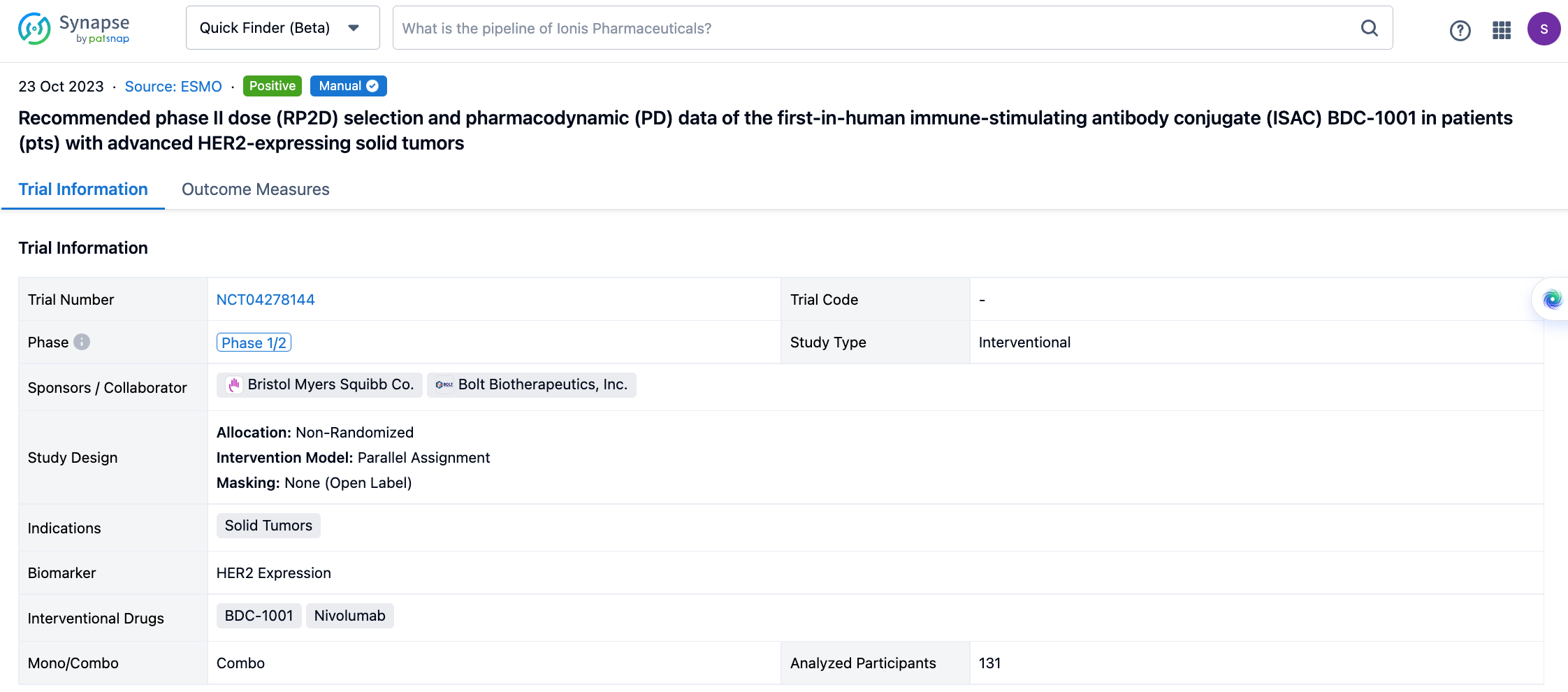
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
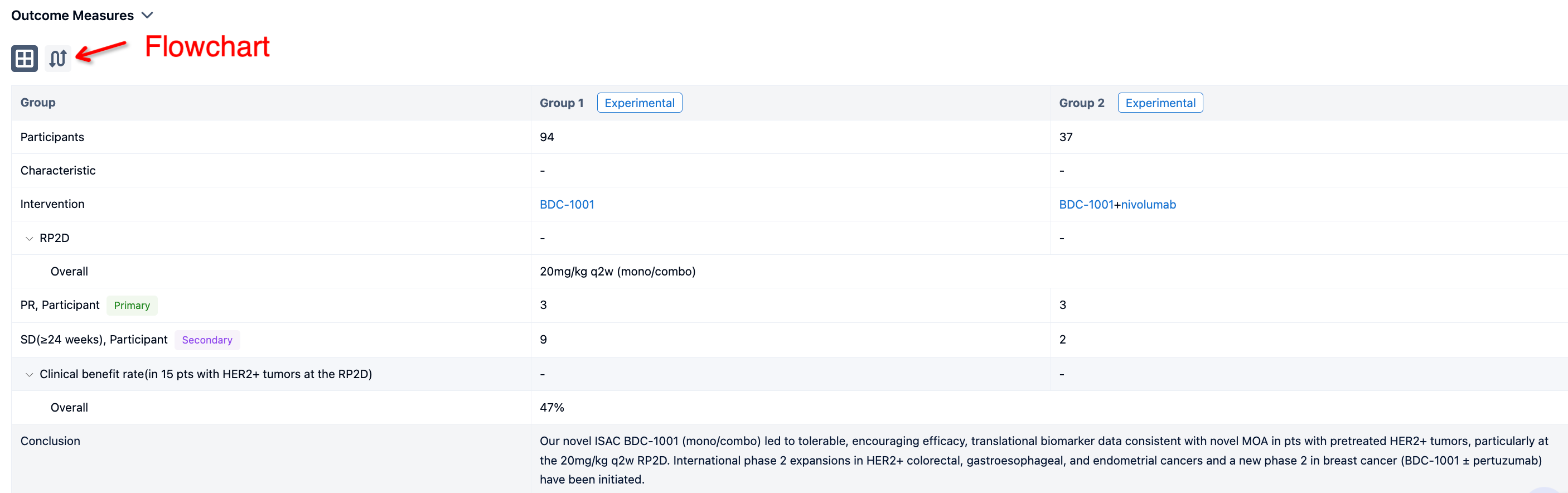
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
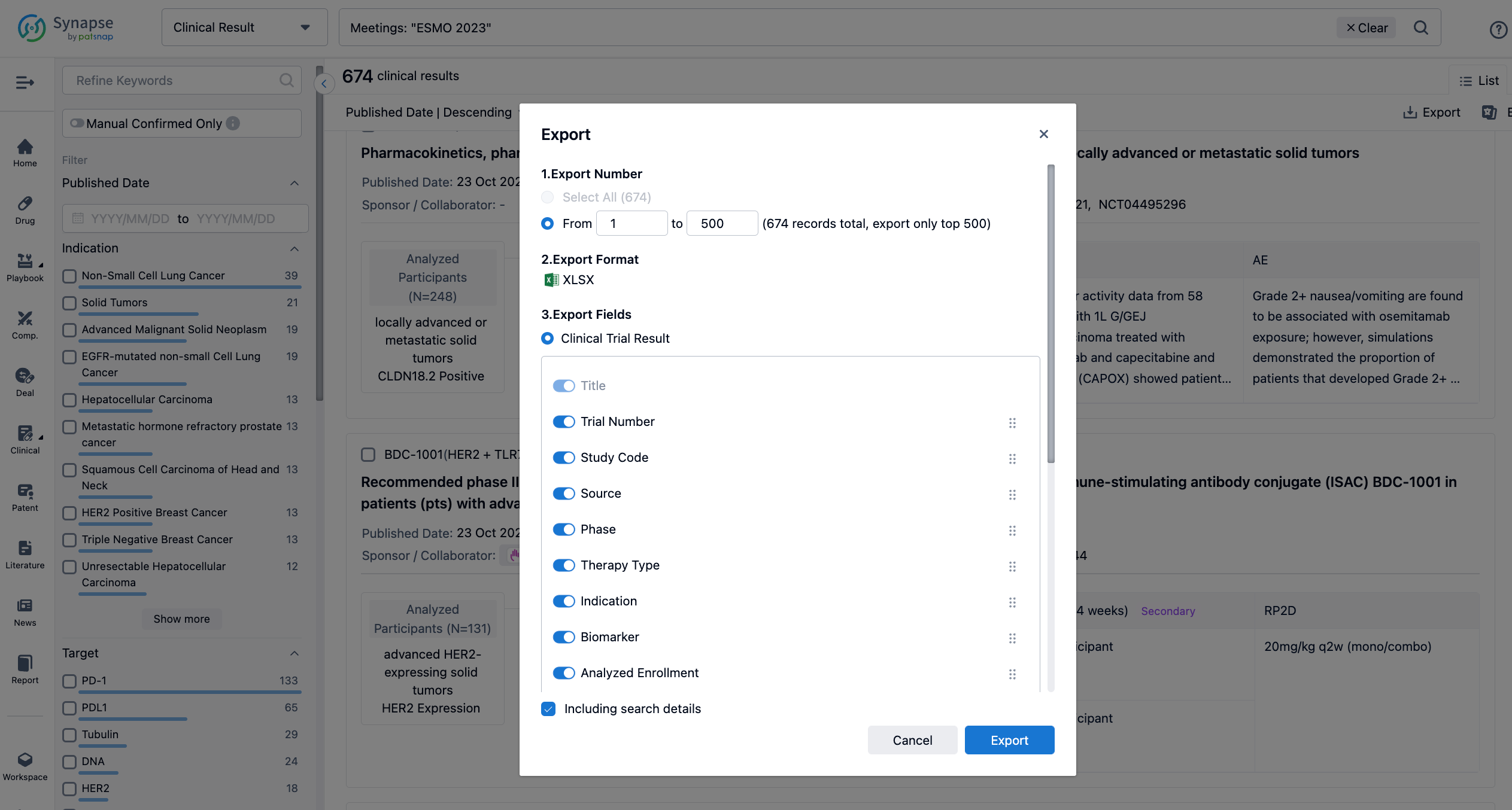
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!