NMPA Approves Henlius' IND Application for Pembrolizumab Biosimilar
Shanghai Henlius Biotech, Inc. (2696.HK) announced that the National Medical Products Administration (NMPA) has approved their investigational new drug (IND) application for the clinical trial of HLX17, a pembrolizumab biosimilar developed independently by the company. This biosimilar will target the same indications as pembrolizumab, including melanoma, non-small cell lung cancer, esophageal cancer, head and neck squamous cell cancer, colorectal cancer, hepatocellular carcinoma, biliary tract cancer, triple-negative breast cancer, microsatellite instability-high or mismatch repair deficient cancer, and gastric cancer, among others.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
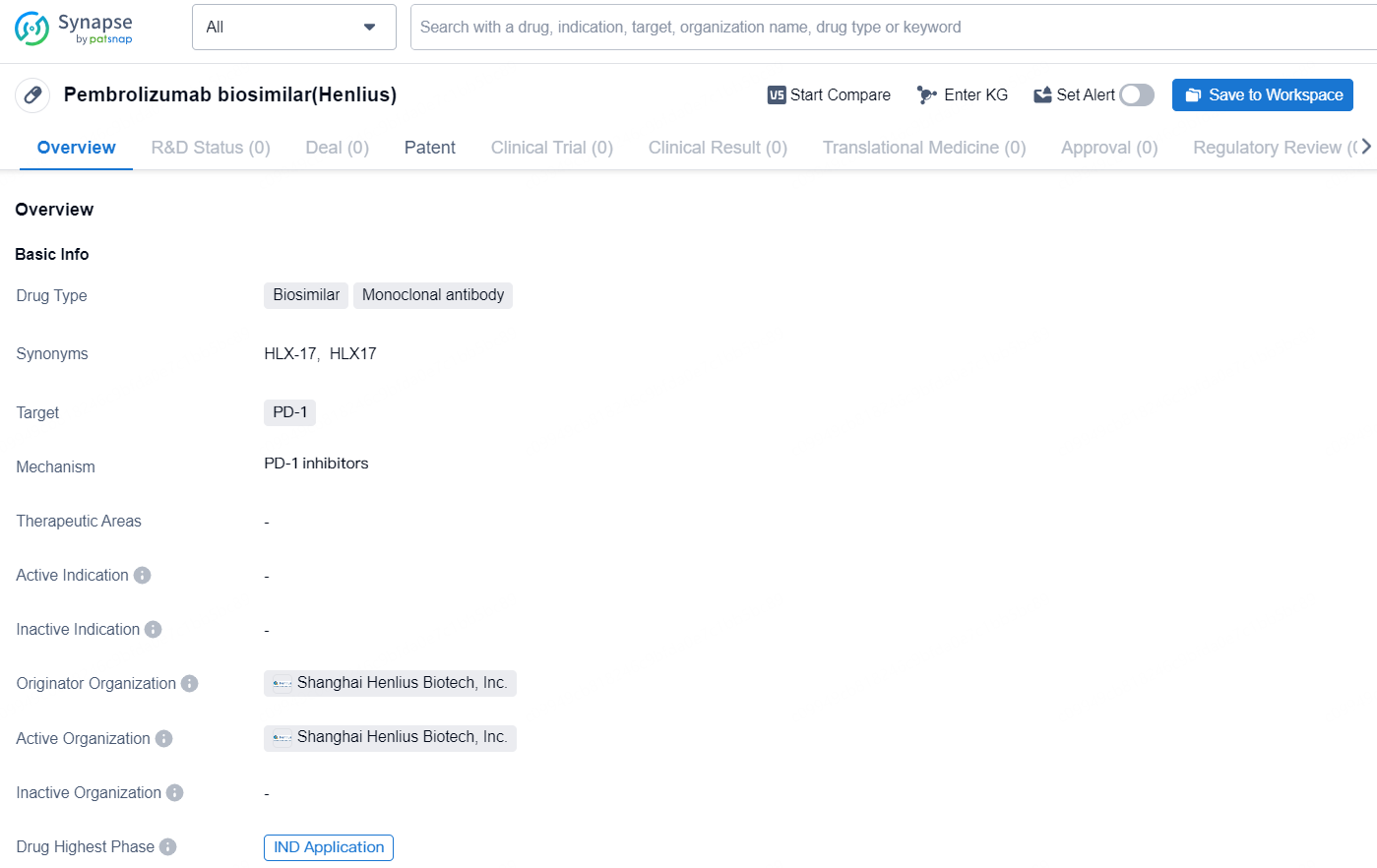
HLX17 is a biosimilar to pembrolizumab, independently developed by Henlius following the biosimilar guiding principles set forth by the NMPA, and in accordance with the guidelines of the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA). Studies on pharmacologic comparisons, preclinical pharmacology, pharmacodynamics, pharmacokinetics, and immunogenicity show that HLX17 has proven comparable to the reference pembrolizumab.
Immune checkpoint inhibitors have become pivotal in immunotherapy, burgeoning as a groundbreaking method against tumor cells, with their unique benefits and significant potential increasingly recognized. HLX17 is a monoclonal antibody designed to bind to the PD-1 receptor on T cells, inhibiting its interaction with PD-L1 and PD-L2. This action lifts PD-1 pathway-mediated suppression of the immune response, including the anti-tumor response, thereby reinstating T-cell immune surveillance over tumors and enhancing its anti-tumor effectiveness.
Leveraging its robust integrated antibody drug R&D platform, Henlius has accelerated the development of immunotherapies and established a diverse pipeline featuring high-potential immune checkpoints like PD-1/L1, CTLA-4, LAG-3, TIGIT, among others. These are anticipated to be effective across various indications and will create synergies with the company's in-house products and other innovative therapies.
Looking ahead, Henlius will continue to focus on unaddressed medical needs, expand its footprint in various disease areas, and commit to delivering high-quality, affordable treatments to patients worldwide.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
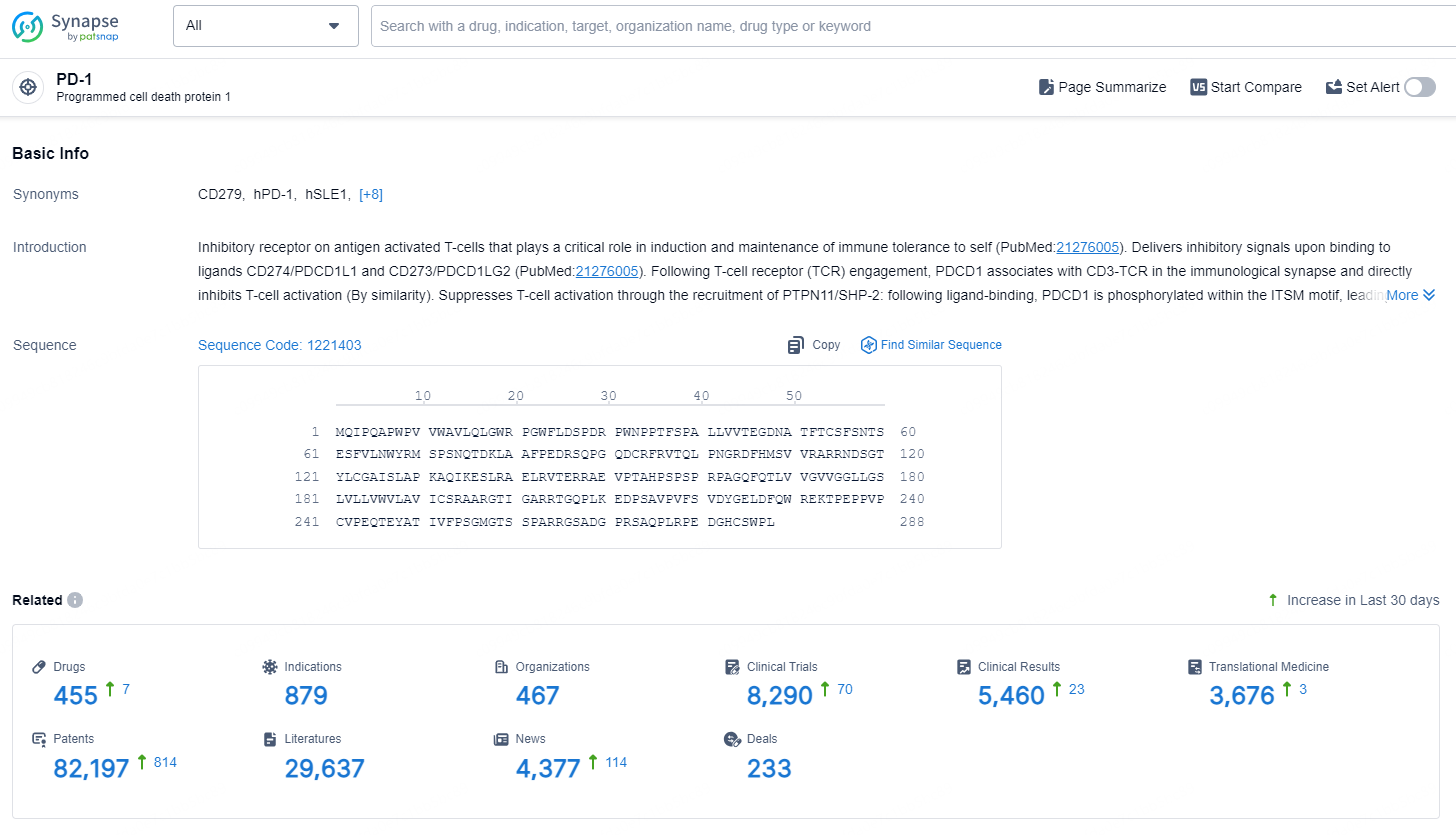
According to the data provided by the Synapse Database, As of September 4, 2024, there are 455 investigational drugs for the PD-1 targets, including 897 indications, 467 R&D institutions involved, with related clinical trials reaching 8290, and as many as 82197 patents.
Pembrolizumab biosimilar (Henlius) is a biosimilar drug classified as a monoclonal antibody, designed to target PD-1. The drug is being developed by Shanghai Henlius Biotech, Inc., with the highest phase of development being the Investigational New Drug (IND) Application in both the global and Chinese markets.