Tolebrutinib Meets Primary Endpoint in HERCULES Phase 3 Study, First to Slow Disability in Non-Relapsing Secondary Progressive MS
The HERCULES phase 3 trial yielded positive outcomes, demonstrating that tolebrutinib, Sanofi’s oral brain-penetrant BTK inhibitor, achieved the primary goal of outperforming placebo in delaying the time to confirmed disability progression (CDP) in individuals with non-relapsing secondary progressive MS (nrSPMS). In the HERCULES trial, nrSPMS was characterized at the start as having an SPMS diagnosis with an expanded disability status scale (EDSS) score ranging from 3.0 to 6.5, no clinical relapses in the prior 24 months, and documented disability accumulation over the previous 12 months. Initial liver safety analysis aligned with findings from earlier tolebrutinib research.
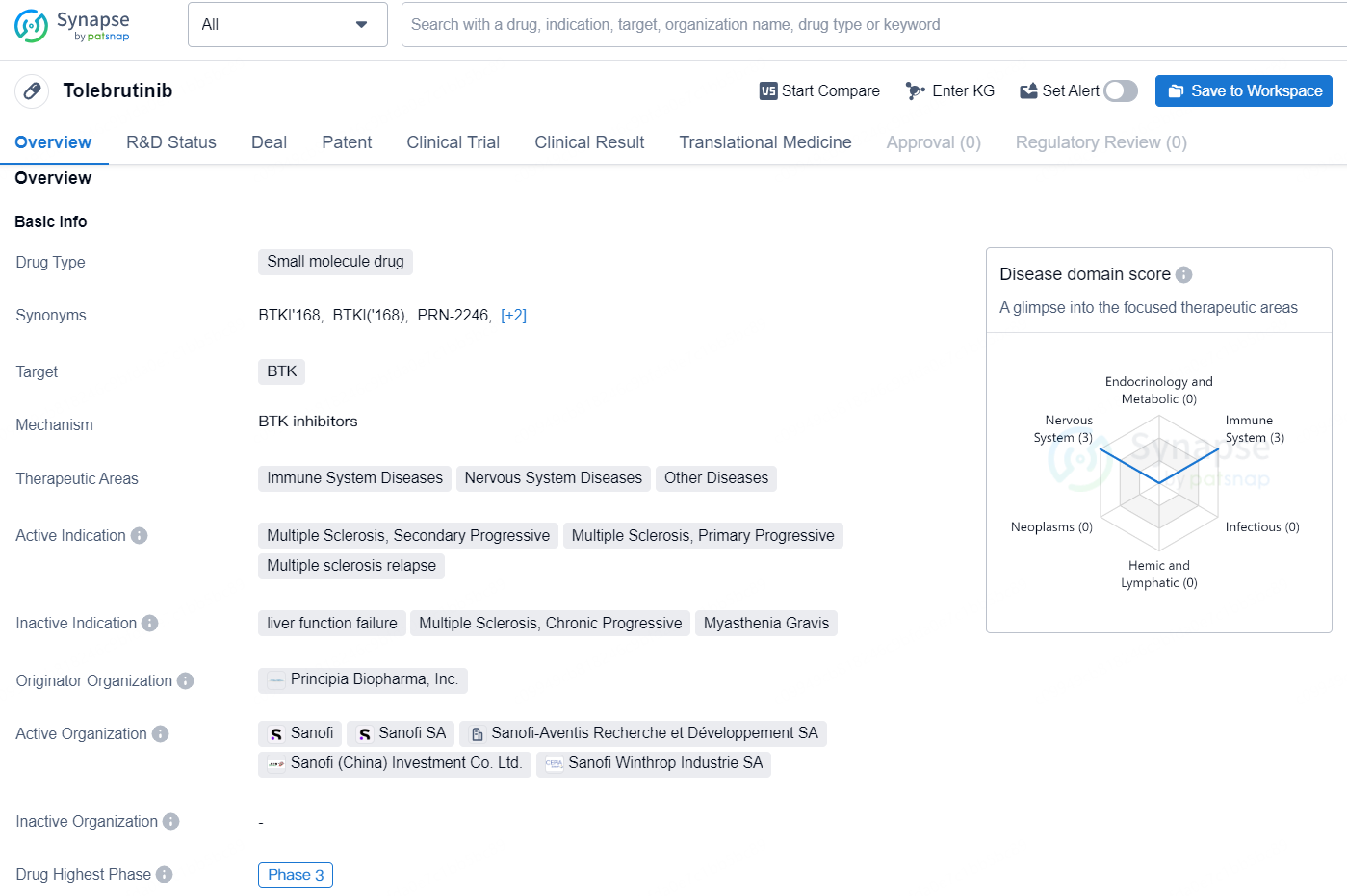
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Findings from the GEMINI 1 and 2 phase 3 trials assessing tolebrutinib failed to achieve the primary goal of lowering the annualized relapse rate (ARR) compared to teriflunomide in individuals with relapsing forms of multiple sclerosis. Nonetheless, evaluation of the combined 6-month CDW data from secondary endpoints revealed a significant delay in onset time, which aligns with the CDP data seen in the HERCULES study.
Dr. Houman Ashrafian, MD, PhD
Research & Development Lead, Sanofi
“Tolebrutinib emerges as a groundbreaking potential first-in-disease treatment with a significant clinical impact on disability progression. Addressing disability progression, thought to be driven by ongoing neuroinflammation, remains the most critical unmet medical need in individuals with non-relapsing secondary progressive MS at present.”
The ongoing PERSEUS phase 3 trial in primary progressive MS focuses on evaluating the time to onset of CDP, with results expected in 2025.
Outcomes from the HERCULES, GEMINI 1, and GEMINI 2 studies will be disclosed at the upcoming ECTRIMS medical conference in Copenhagen, Denmark, on September 20, 2024. Tolebrutinib is still under clinical investigation, and its safety and efficacy have yet to be reviewed by any regulatory body.
Multiple sclerosis is an enduring, immune-mediated, neurodegenerative condition leading to irreversible disability accumulation over time. This physical and cognitive disability progressively worsens the health status and degrades the quality of life, affecting patient care and life expectancy.
Disability progression is the main unmet need in MS treatment. Current therapies primarily target peripheral B and T cells, while the innate immune response—believed to drive disability progression—remains largely unaddressed. Approved therapies or those under investigation primarily target the adaptive immune system and often do not directly affect the central nervous system (CNS) to confer clinical advantages.
RMS refers to individuals with MS who encounter episodes of new or worsening symptoms (relapses) followed by periods of partial or full recovery. nrSPMS refers to individuals with MS who no longer experience confirmed relapses but continue to accumulate disability, observed as symptoms including fatigue, cognitive impairment, balance and gait issues, loss of bowel and/or bladder function, and sexual dysfunction among others.
Tolebrutinib's mechanism modulates both B lymphocytes and activated microglia within the CNS, believed to address the core mechanisms of disability progression in MS associated with ongoing neuroinflammation in the brain and spinal cord.
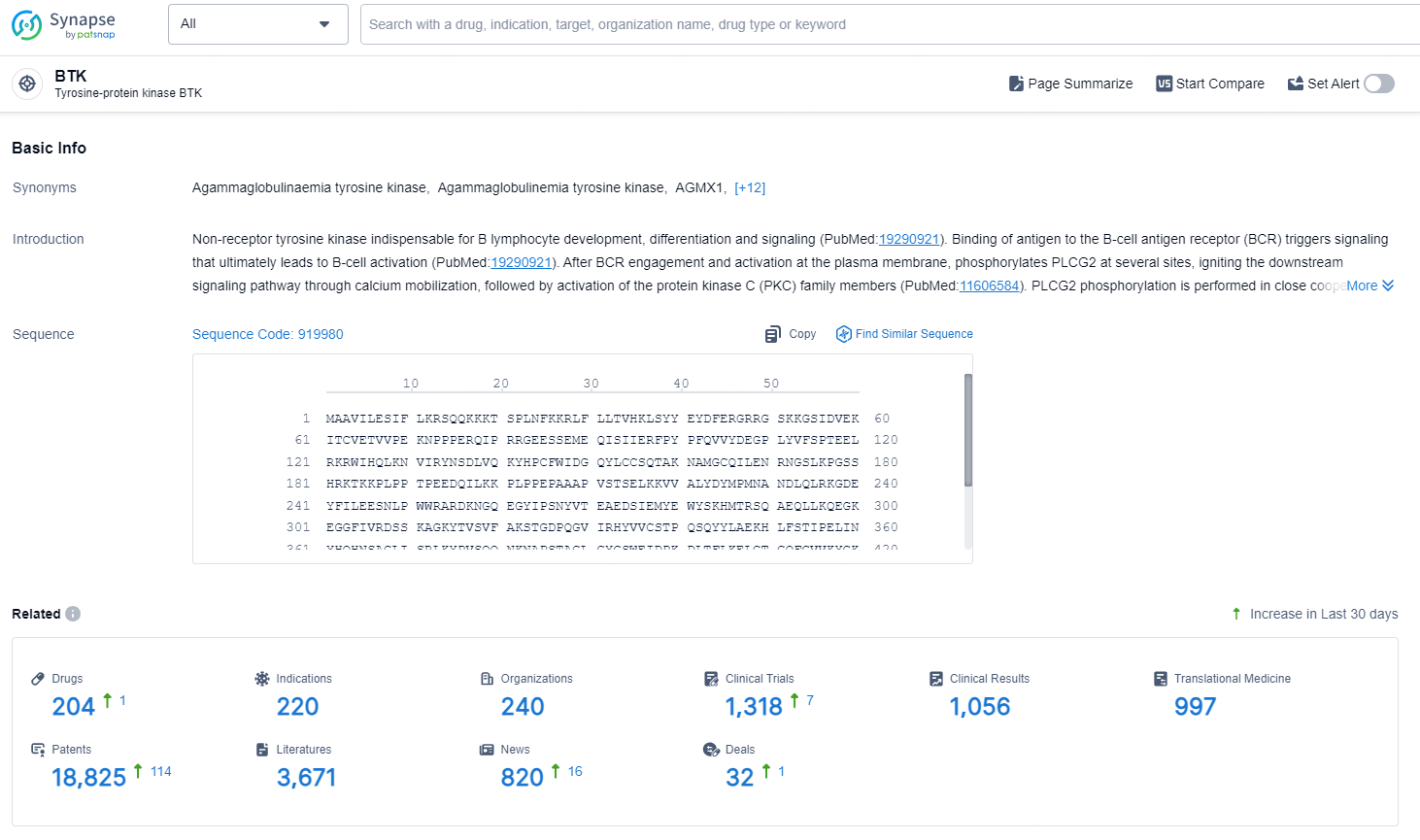
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 3, 2024, there are 204 investigational drugs for the BTK targets, including 220 indications, 240 R&D institutions involved, with related clinical trials reaching 1318, and as many as 18825 patents.
Tolebrutinib is a small molecule drug that targets the Bruton's tyrosine kinase (BTK). It is being developed for the treatment of immune system diseases, nervous system diseases, and other diseases. The drug is currently in the highest phase of clinical development, which is Phase 3, both globally and in China. Tolebrutinib is being investigated for its potential in treating multiple sclerosis, including secondary progressive, primary progressive, and relapsing forms of the disease. It is being developed by Principia Biopharma, Inc., a biopharmaceutical company specializing in developing novel small molecule therapeutics.