NMPA Approves HLX53 Anti-TIGIT Trial with HANSIZHUANG/HANBEITAI for Advanced Liver Cancer
Shanghai Henlius Biotech, Inc. has declared that the National Medical Products Administration has greenlit the investigational new drug application for its clinical study. This trial will explore the efficacy of HLX53, an anti-TIGIT Fc fusion protein, when used together with HANSIZHUANG (serplulimab, HLX10) and HANBEITAI (bevacizumab, HLX04) in the initial treatment of patients suffering from either locally advanced or metastatic hepatocellular carcinoma.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Liver cancer ranks among the top cancers globally in terms of incidence and mortality. As per GLOBALCAN 2022 statistics, around 870,000 new cases and 760,000 deaths are associated with this malignancy worldwide each year. In China, primary liver cancer is the fourth most diagnosed and the second leading cause of cancer death, contributing to approximately 370,000 new cases and 320,000 fatalities in 2022.
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, comprising 85% to 90% of all liver cancer cases. Its stealthy nature, often asymptomatic early stages, and rapid disease progression mean that many patients are only diagnosed at an advanced stage, which complicates treatment and adversely affects outcomes. The five-year survival rate for patients with PLC hovers around 18%.
The go-to approach for treating advanced stages of liver cancer involves targeted therapy and immunotherapy, which have demonstrated significant efficacy in controlling the disease and extending patient survival. Despite these advancements, recurrence and disease progression still occur in some patients. There is a pressing need to broaden the pool of patients who can benefit from these advanced therapies and to enhance the effectiveness of treatments for liver cancer.
HLX53 is an innovative anti-TIGIT Fc fusion protein developed by Henlius. This compound combines the variable domain of the heavy chain from a heavy-chain antibody with a wildtype IgG1 Fc segment. Early laboratory studies show that HLX53 has strong anti-tumor properties and a favorable safety profile. Henlius has also commenced a phase 1 clinical trial to assess the safety, tolerability, pharmacokinetics, and initial effectiveness of HLX53 in treating patients with advanced or metastatic solid tumors or lymphomas who have not responded to or are ineligible for standard therapies.
Given the effective results of the immune checkpoint inhibitor pairing of anti-PD-1/PD-L1 antibodies with anti-angiogenic drugs in first-line treatments for advanced HCC, and considering the synergistic potential of combining TIGIT and PD-1/PD-L1 pathways, Henlius is exploring the integration of TIGIT inhibitors into standard treatment protocols to potentially enhance clinical outcomes for patients with advanced HCC.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
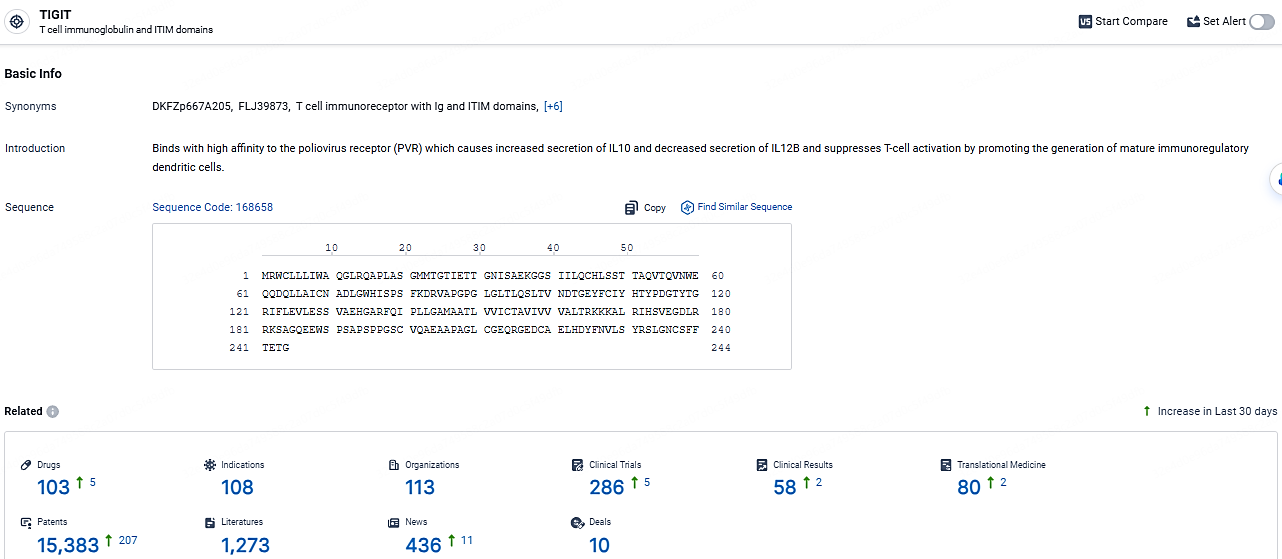
According to the data provided by the Synapse Database, As of April 22, 2024, there are 103 investigational drugs for the TIGIT target, including 108 indications, 113 R&D institutions involved, with related clinical trials reaching 286, and as many as 15383 patents.
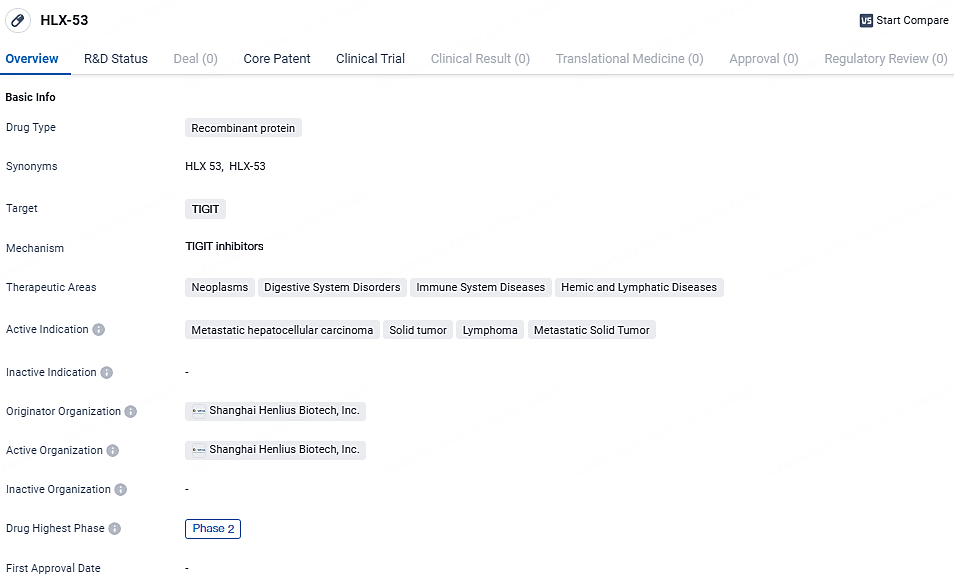
HLX-53 is a recombinant protein drug targeting TIGIT, with a focus on treating various types of cancers and other related diseases. Its current development stage is Phase 2 globally and Phase 1 in China. The drug's potential therapeutic benefits and its originator organization's active involvement in its development highlight the significance of HLX-53 in the field of biomedicine.