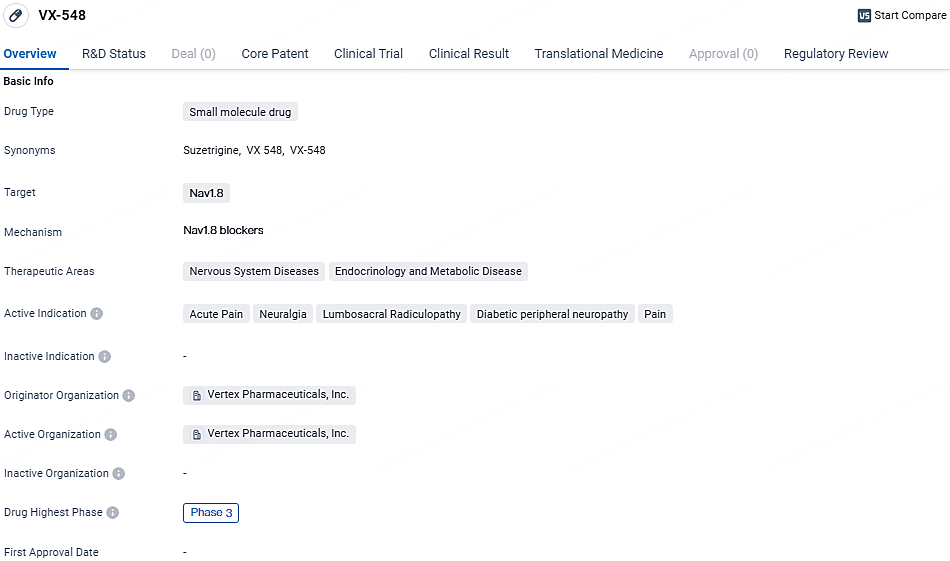
Vertex Reveals Progress in Suzetrigine (VX-548) for Acute and Neuropathic Pain Treatment
Vertex Pharmaceuticals Incorporated has disclosed significant progress in its suzetrigine-based pain management program. Suzetrigine, an oral selective NaV1.8 pain signal inhibitor previously identified as VX-548, is poised to become the inaugural class of analgesics for both acute and neuropathic pain, marking the first such development in over twenty years.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Following the encouraging outcomes from Phase 3 trials for acute pain reported in January 2024, the Food and Drug Administration approved an ongoing New Drug Application for suzetrigine for treating moderate-to-severe acute pain. Vertex launched this rolling application process, anticipating completion by the second quarter of 2024. Previously, suzetrigine received FDA Fast Track and Breakthrough Therapy designations for this same level of acute pain.
Concerning neuropathic pain, Vertex publicized favorable results from its Phase 2 trial in December 2023 and recently concluded a productive end-of-Phase 2 discussion with the FDA. Vertex is gearing up to start a Phase 3 pivotal study of suzetrigine in Diabetic Peripheral Neuropathy (DPN) patients in the second half of 2024. Suzetrigine has also been awarded a Breakthrough Therapy designation by the FDA for its application in treating pain linked with DPN.
The upcoming Phase 3 studies will consist of two 12-week randomised, double-blind, placebo-controlled trials to assess the effectiveness and safety of suzetrigine in DPN patients. The principal goal for these studies is measuring the alteration from the start in the average weekly pain intensity using the numeric pain rating scale at Week 12, in comparison to a placebo. Each study will measure a critical secondary endpoint: the difference in average weekly pain intensity at Week 12 against pregabalin.
It is anticipated that around 1,100 participants will join each of these Phase 3 trials. Participants completing these controlled trials have the opportunity to join an open-label trial to further examine suzetrigine’s long-term safety and efficacy in DPN.
Furthermore, Vertex is actively enrolling patients for its ongoing Phase 2 trial of suzetrigine in individuals with lumbosacral radiculopathy, which is due to disturbances or damage to nerve roots in the lumbar spine region. The recruitment for this study is expected to be concluded by year-end.
"This represents a crucial step forward in our mission to transform pain management," commented Carmen Bozic, M.D., Executive Vice President, Global Medicines Development and Medical Affairs, and Chief Medical Officer at Vertex. "With suzetrigine's demonstrated favorable benefit/risk ratio throughout our clinical trials and positive regulatory feedback, we are optimistic about the potential of this innovative non-opioid treatment to significantly help countless patients dealing with both acute and neuropathic pain."
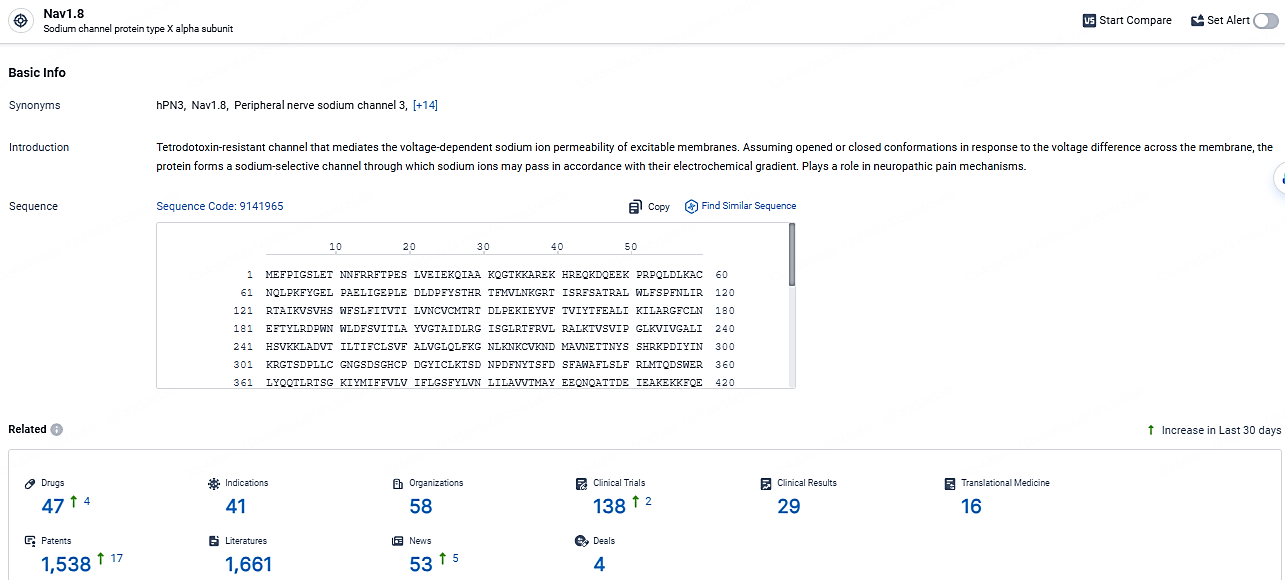
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 22, 2024, there are 47 investigational drugs for the NaV1.8 target, including 41 indications, 58 R&D institutions involved, with related clinical trials reaching 138, and as many as 1538 patents.
VX-548's focus on Nav1.8 as a target suggests its potential in modulating pain signals in the nervous system. The drug's indications cover a range of pain conditions, including acute and chronic pain associated with nerve damage. The fact that VX-548 is in Phase 3 indicates that it has progressed through earlier stages of development and is closer to potential approval. The regulatory designations of Fast Track and Breakthrough Therapy highlight the drug's potential to address unmet medical needs and improve patient outcomes.