Norfloxacin: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Norfloxacin's R&D Progress
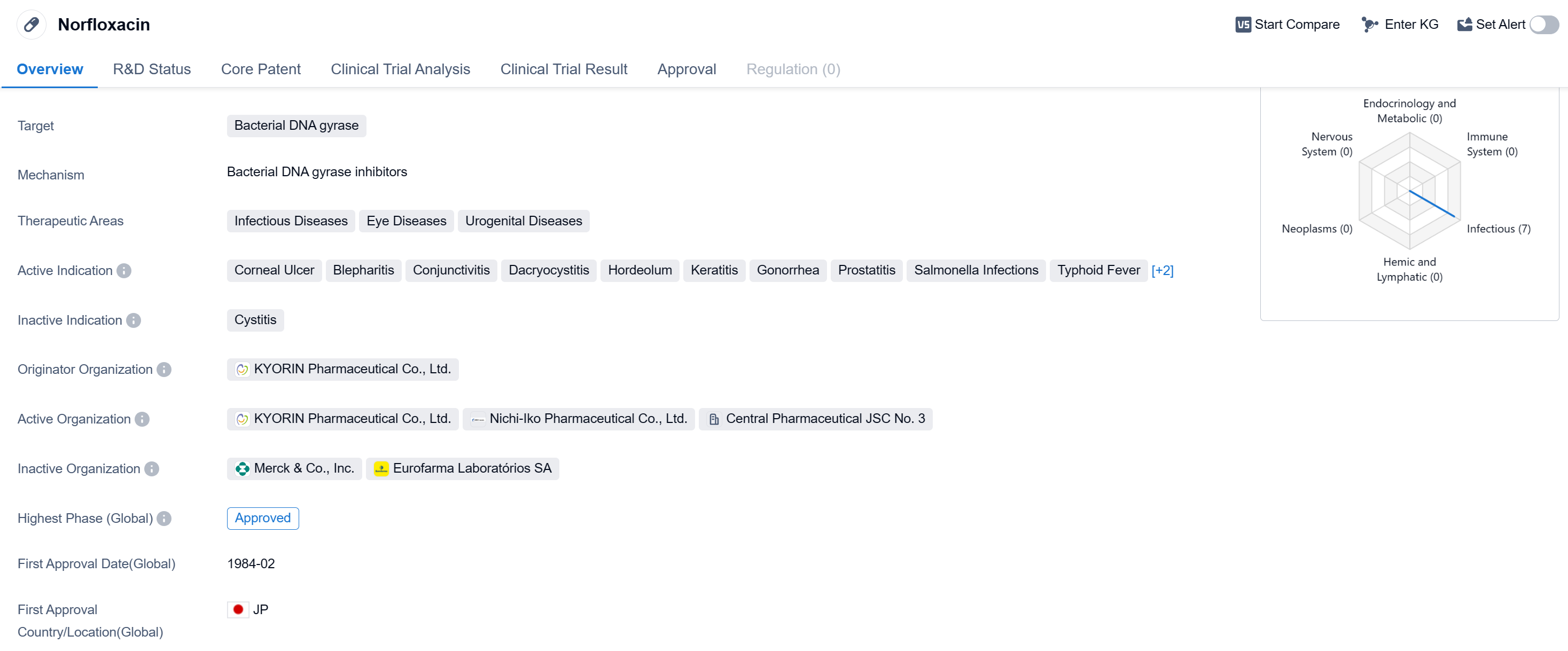
Norfloxacin is a small molecule drug that belongs to the class of fluoroquinolones. It primarily targets bacterial DNA gyrase, an enzyme involved in the replication and repair of bacterial DNA. This drug has shown efficacy in treating various infectious diseases, eye diseases, and urogenital diseases.
In terms of therapeutic areas, Norfloxacin has been approved for the treatment of infectious diseases, eye diseases, and urogenital diseases. Specifically, it has been indicated for the treatment of corneal ulcer, blepharitis, conjunctivitis, dacryocystitis, hordeolum, keratitis, gonorrhea, prostatitis, salmonella infections, typhoid fever, urinary tract infections, and other infectious diseases.
Norfloxacin was first approved in Japan in February 1984 by KYORIN Pharmaceutical Co., Ltd., making it one of the early drugs in the fluoroquinolone class. It has since received approvals in other countries as well, indicating its global recognition and acceptance.
As a small molecule drug, Norfloxacin is designed to be easily absorbed and distributed throughout the body. Its mechanism of action involves inhibiting the activity of bacterial DNA gyrase, which ultimately leads to the disruption of bacterial DNA replication and cell division. By targeting this essential bacterial enzyme, Norfloxacin effectively combats bacterial infections.
The approval of Norfloxacin in multiple therapeutic areas highlights its versatility and broad-spectrum activity against various bacterial pathogens. Its efficacy in treating eye diseases, urogenital diseases, and infectious diseases makes it a valuable option for healthcare professionals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Norfloxacin: Bacterial DNA gyrase inhibitors
Bacterial DNA gyrase inhibitors are a type of drugs that target and inhibit the activity of DNA gyrase, an enzyme found in bacteria. DNA gyrase is responsible for the supercoiling of bacterial DNA, which is essential for DNA replication and transcription. By inhibiting DNA gyrase, these inhibitors interfere with the bacterial DNA replication process, ultimately leading to the inhibition of bacterial growth and reproduction.
From a biomedical perspective, bacterial DNA gyrase inhibitors are important in the field of antimicrobial therapy. They are commonly used as antibiotics to treat bacterial infections. By specifically targeting the DNA gyrase enzyme in bacteria, these inhibitors selectively kill or inhibit the growth of bacteria while minimizing harm to human cells. This makes them effective in treating various bacterial infections, including respiratory tract infections, urinary tract infections, and skin infections.
Examples of bacterial DNA gyrase inhibitors include fluoroquinolones such as ciprofloxacin, levofloxacin, and moxifloxacin. These drugs work by binding to the DNA gyrase enzyme and preventing it from carrying out its normal function. As a result, bacterial DNA replication is disrupted, leading to the eventual death or inhibition of the bacteria.
It is important to note that bacterial DNA gyrase inhibitors should be used under the guidance of a healthcare professional, as their misuse or overuse can contribute to the development of antibiotic resistance.
Drug Target R&D Trends for Norfloxacin
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 186 Bacterial DNA gyrase drugs worldwide, from 216 organizations, covering 164 indications, and conducting 1205 clinical trials.
The analysis of the current competitive landscape of the target Bacterial DNA gyrase reveals that Novartis AG is the company with the highest number of approved drugs. The indication with the highest number of approved drugs is Bacterial Infections. Small molecule drugs are progressing most rapidly, followed by bacteriophage therapy. China, Japan, United States, and the European Union are the countries/locations with the highest number of approved drugs. The future development of the target Bacterial DNA gyrase is expected to continue with a focus on small molecule drugs and further research in countries like China, Japan, United States, and the European Union.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Norfloxacin is a well-established drug that has been approved for use in Japan and globally. Its long history of use and multiple indications demonstrate its effectiveness in combating bacterial infections. As an expert in the pharmaceutical industry, it is important to consider Norfloxacin's therapeutic potential and its role in addressing the medical needs of patients with infectious diseases, eye diseases, and urogenital diseases.