Novartis Reveals Zolgensma Data Improves Motor Skills in Older SMA Children
Novartis has unveiled recent findings endorsing the efficacy of Zolgensma® (onasemnogene abeparvovec) as a unique single-administration gene treatment for managing spinal muscular atrophy. These results further substantiate the therapeutic advantages of this approach.
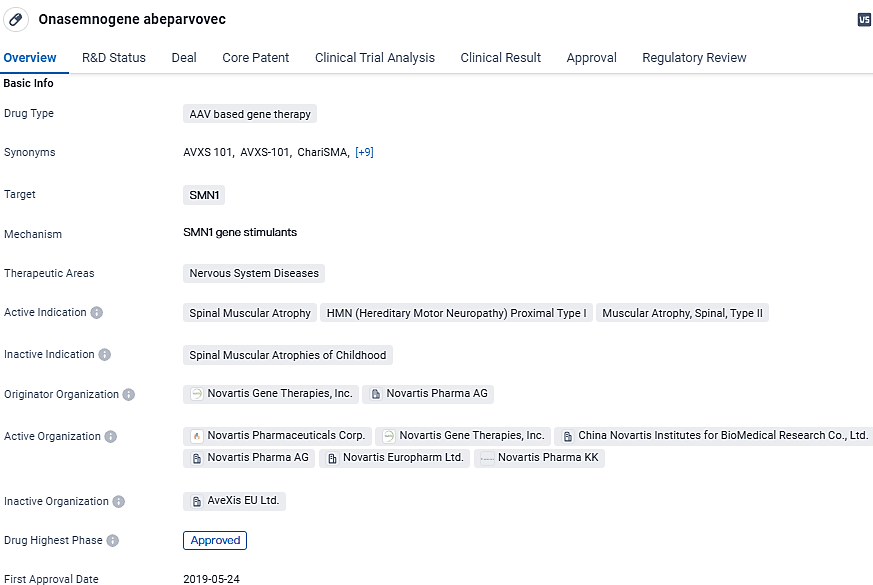
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Recent findings from the SMART research illuminate the robust safety and efficacy profile of Zolgensma for children with Spinal Muscular Atrophy (SMA) whose body weight ranges from above 8.5 kg to below or equal to 21 kg. These participants had an average age of 4.69 years, and it is noteworthy that the majority had ceased using an alternative disease-modifying treatment prior to receiving Zolgensma.
The latest scientific outcomes provide additional insight into the real-world applications of this cutting-edge therapy in older pediatric patients with more substantial body weight in regions where its use is not limited by patient age. The information presented here is part of a larger compilation of Zolgensma data showcased at the 2024 Muscular Dystrophy Association Clinical and Scientific Conference, taking place in Orlando, Florida, from March 3rd to 6th.
Pediatric Neurologist Dr. Hugh McMillan shared his perspective: "The insights gained from the SMART trial underscore Zolgensma's clinical benefits for more mature and heavier children affected by SMA, especially those with a history of other disease-modifying treatments." Dr. McMillan emphasized that these findings lend support to the administration of Zolgensma for children up to 21 kg and endorse the gene-replacement therapy as a viable treatment path for a more inclusive patient demographic.
The SMART study's fundamental aim was to gauge the safety and acceptability of Zolgensma for patient groups beyond those included in earlier trials in terms of age and weight. A common outcome among participants was elevated transaminase levels and a temporary drop in platelet count. Nevertheless, these occurrences were asymptomatic and addressed through vigilant oversight and management in line with the recommended guidelines included in the product information. The study did not present any previously unknown safety concerns.
Dr. Sandra P. Reyna, Novartis' Chief Scientific Advisor and Head of Global Medical Engagement for SMA, made the following assertion: "The data collected through this first open-label Zolgensma study, encompassing both older and heavier patients as well as those previously treated, is expected to instill confidence in caregivers and healthcare providers. It aids them in making well-informed treatment choices that are in alignment with the guidance provided on the local product's label for the respective patient group."
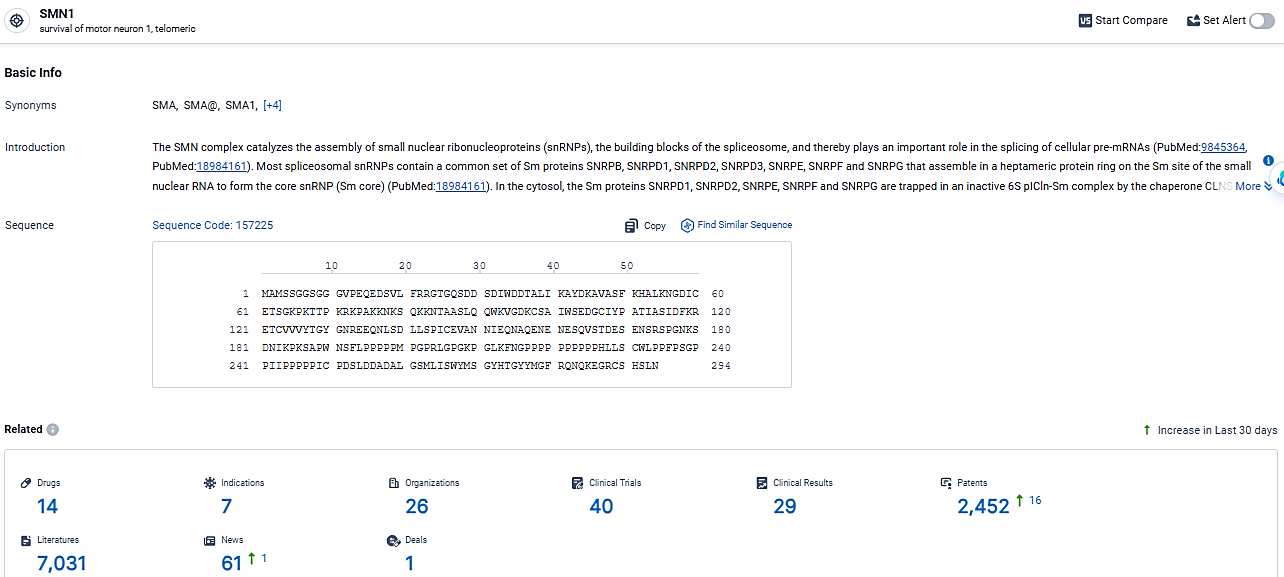
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 5, 2024, there are 14 investigational drugs for the SMN1 target, including 7 indications,26 R&D institutions involved, with related clinical trials reaching 40, and as many as 2452 patents.
Onasemnogene abeparvovec targets the SMN1 gene and has received regulatory approval in the United States for the treatment of SMA. With its potential to address other related conditions and its breakthrough therapy designation, the drug represents a significant advancement in the field of biomedicine and offers hope for patients suffering from these debilitating nervous system diseases.