Numab Therapeutics Initiates Phase 1 Trial for NM32 in Solid Tumors
Numab Therapeutics AG ("Numab") has unveiled the initiation of a Phase 1 clinical study for NM32, a novel tri-specific immuno-oncology agent designed to target the receptor tyrosine kinase-like orphan receptor 1 (ROR1) as well as the CD3 antigen associated with T-cell receptors. The unique third binding component of NM32 is directed at serum albumin, which serves to extend its half-life, facilitating bi-weekly administration, while maintaining a lower molecular weight than that of immunoglobulins to enhance tumor infiltration efficiency.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Developed using our exclusive Lambda-capTM and MATCHTM technology platforms, NM32 functions as a T-cell engager aimed at enhancing the immune system's ability to identify and destroy tumor cells. ROR1 is significantly overexpressed in a range of common solid tumor types while showing minimal presence in normal tissues, which positions it as an ideal target for a CD3 engager, according to David Urech, Ph.D., Founder and Chief Executive Officer of Numab Therapeutics.
“We are excited to embark on the clinical development of NM32, which may provide this innovative therapy to patients suffering from advanced solid tumors who require treatment options with sustained anti-tumor efficacy and manageable safety. Patient enrollment is proceeding well, and individuals are currently finishing the initial treatment cycle at the first dose level,” stated Martin Stern, Senior Vice President of Clinical Science at Numab Therapeutics. “Although checkpoint inhibitors and various CD3 engagers for hematologic cancers have been introduced, the results in solid tumors have been limited primarily due to the shortage of highly specific tumor antigens that facilitate targeted T-cell activation against the tumor.”
This Phase 1 multi-center study of NM32 is designed as a dose-escalation trial aimed at evaluating its pharmacokinetics, pharmacodynamics, and safety profile, as well as determining the most effective dosing schedule for subsequent clinical trials. The study plans to enroll up to 60 patients with solid tumors that overexpress ROR1, sourced from key clinical centers throughout the United States. Preclinical findings in non-human primate models have demonstrated strong efficacy and a favorable safety record.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
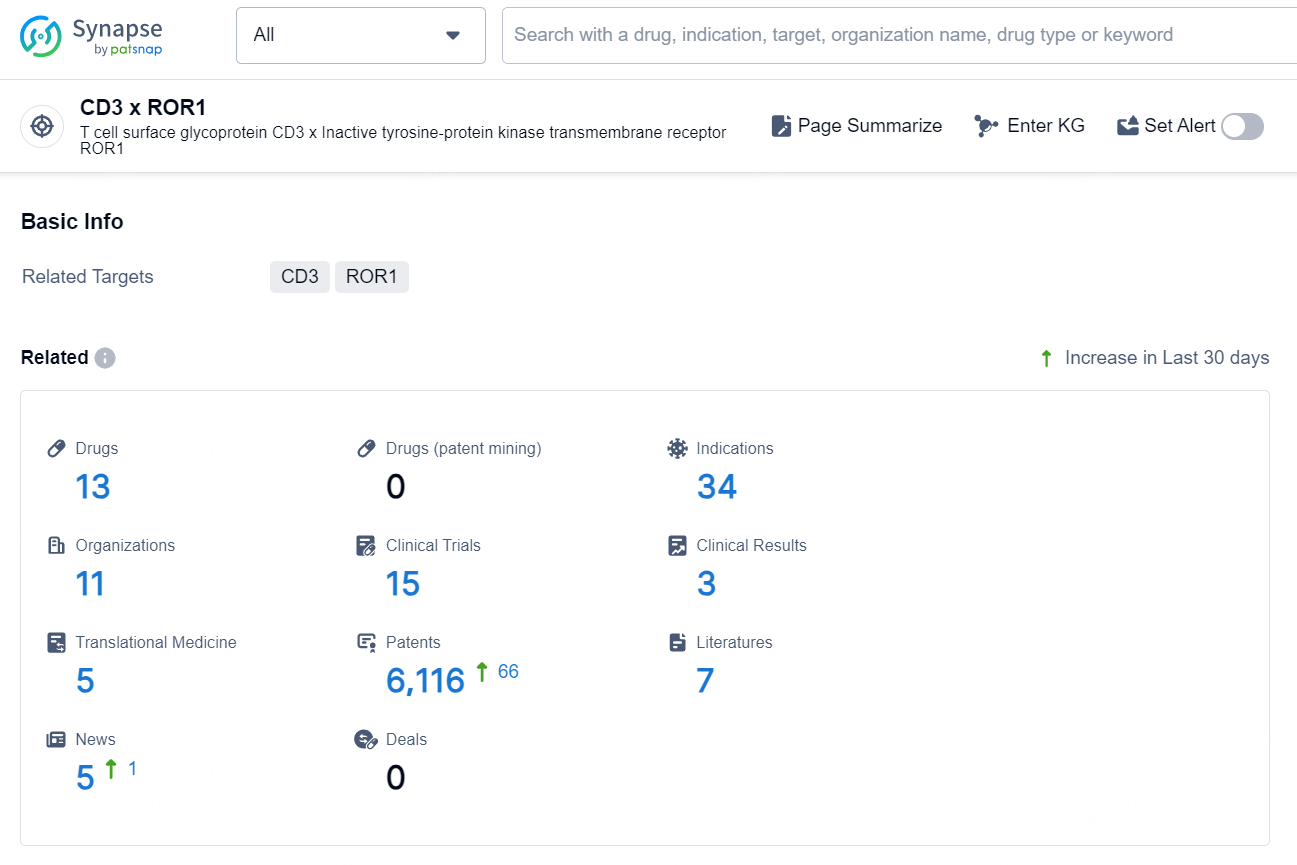
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 13 investigational drugs for the CD3 x ROR1 target, including 34 indications, 11 R&D institutions involved, with related clinical trial reaching 15, and as many as 6116 patents.
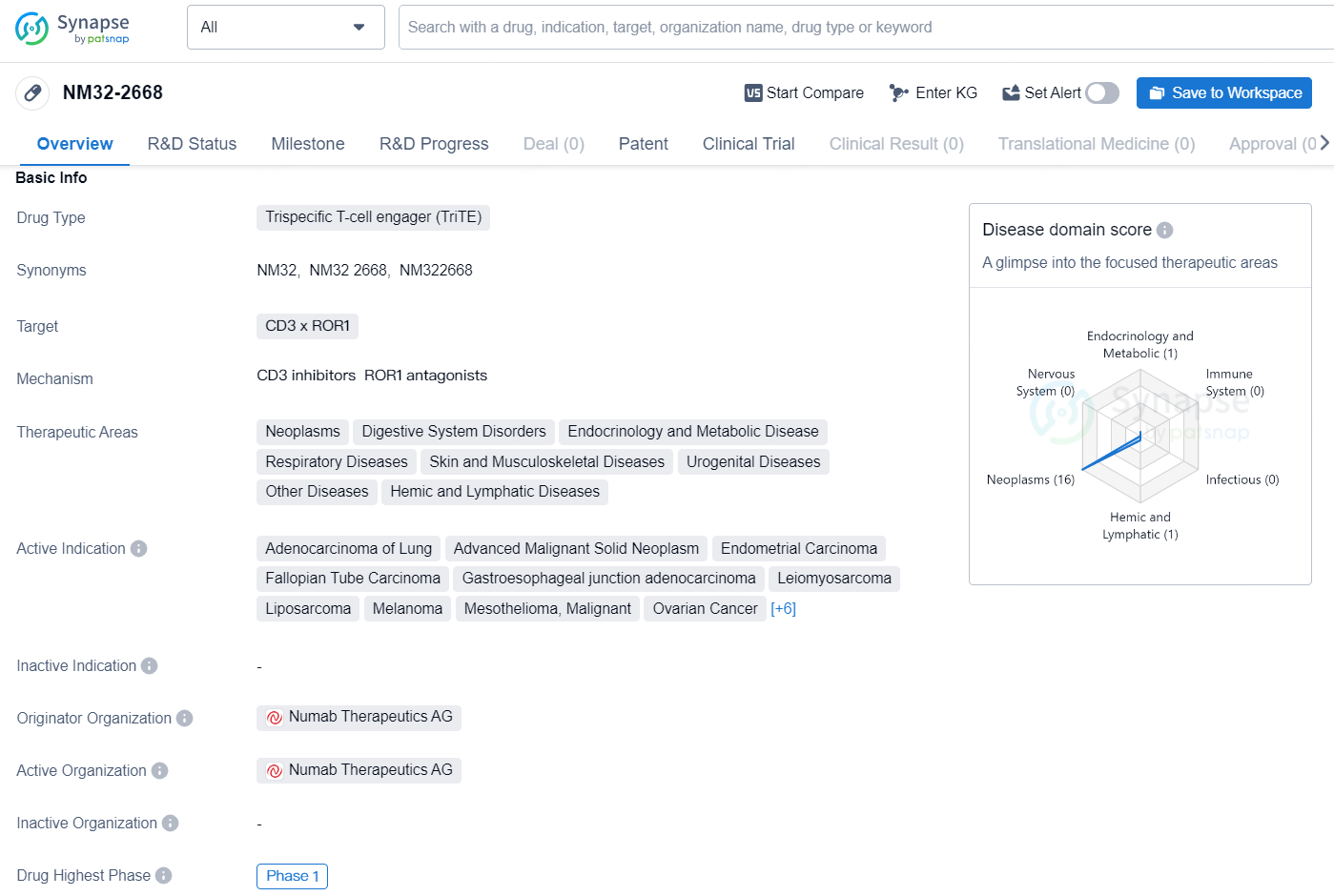
NM32-2668 is a trispecific T-cell engager (TriTE) drug that targets CD3 x ROR1. It is being developed to treat a wide range of therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, and hemic and lymphatic diseases.