Icovamenib Enhances GLP-1 Therapy Efficacy: Biomea Fusion's Preclinical Findings
Biomea Fusion, Inc. (Nasdaq: BMEA), a biopharmaceutical firm in the clinical development phase focusing on the creation of oral covalent small molecules aimed at enhancing the well-being of individuals suffering from diabetes, obesity, and genetically-driven cancers, unveiled preclinical findings indicating that icovamenib improved the effectiveness of GLP-1-based treatments. Additionally, early preclinical efficacy and pharmacokinetic results for BMF-650, a next-generation oral small-molecule GLP-1 RA receptor agonist candidate, were also shared.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Icovamenib (BMF-219) Combined with GLP-1-Based Therapy
Key Insights:
Preclinical investigations assessed insulin release via GLP-1-based treatments (tirzepatide and semaglutide) utilizing human islets cultivated in ex vivo hyperglycemic environments, both with and without icovamenib intervention.
We posited that inhibiting menin could amplify the effects of GLP-1-based treatments. The data indicated enhanced insulin responses for both tirzepatide and semaglutide when paired with icovamenib.
Additional studies involving orforglipron suggested that prior treatment with icovamenib led to a notable increase in insulin secretion, nearly doubling the effectiveness based on dosages used.
Moreover, BMF-650, Biomea's innovative oral small molecule GLP-1 receptor agonist candidate, showed superior outcomes whether administered alone or in conjunction with icovamenib, underscoring its promising therapeutic advantages.
A Phase II trial, COVALENT-211, is set to start in 2025 to assess the efficacy of combining icovamenib with GLP-1-based therapies. The Company also provided further insights into its investigational next-generation oral GLP-1 RA candidate, BMF-650.
BMF-650: Investigational Next-Generation Oral Small Molecule GLP-1 Receptor Agonist – Key Findings:
Preclinical analysis was performed to contrast the characteristics of BMF-650 against a leading oral GLP-1 RA. BMF-650 demonstrated enhanced bioavailability and a more consistent pharmacokinetic profile, potentially leading to improved tolerance and facilitating effective dose escalation for patients. The projected human dosage is approximately 100 mg once daily.
In studies involving human donor islets, BMF-650 notably improved glucose-stimulated insulin secretion.
Cynomolgus monkey trials corroborated these findings, indicating significant enhancements in glucose-stimulated insulin release aligned with results from human studies. Additionally, BMF-650 exhibited superior glucose regulation.
Investigations on appetite suppression indicated that daily administration of BMF-650 markedly reduced food consumption during peak drug levels, with sustained effects observed over a six-day evaluation period.
These results emphasize BMF-650's promise as an oral intervention for diabetes and obesity.
“Our preclinical observations on menin inhibition within the GLP-1 pathway may enhance the profile of icovamenib as a potential adjunct to GLP-1-based therapies. The use of icovamenib may allow for reduced dosages of GLP-1 RA, thereby maximizing the overall therapeutic benefits. We anticipate icovamenib will improve efficacy, tolerability, and adherence, ultimately providing longer-term advantages for more patients utilizing these treatments,” stated Thomas Butler, Chief Executive Officer and Chairman of Biomea Fusion. “We are equally enthusiastic about the preliminary outcomes of BMF-650, which have showcased distinct benefits compared to a leading GLP-1 RA in our preclinical evaluations. BMF-650 has exhibited superior insulin secretion, enhanced glucose management, a more stable pharmacokinetic profile, and increased bioavailability, all suggesting a broader therapeutic potential. The appetite suppression effects are particularly promising, indicating a novel and significant profile that could positively influence obesity outcomes.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
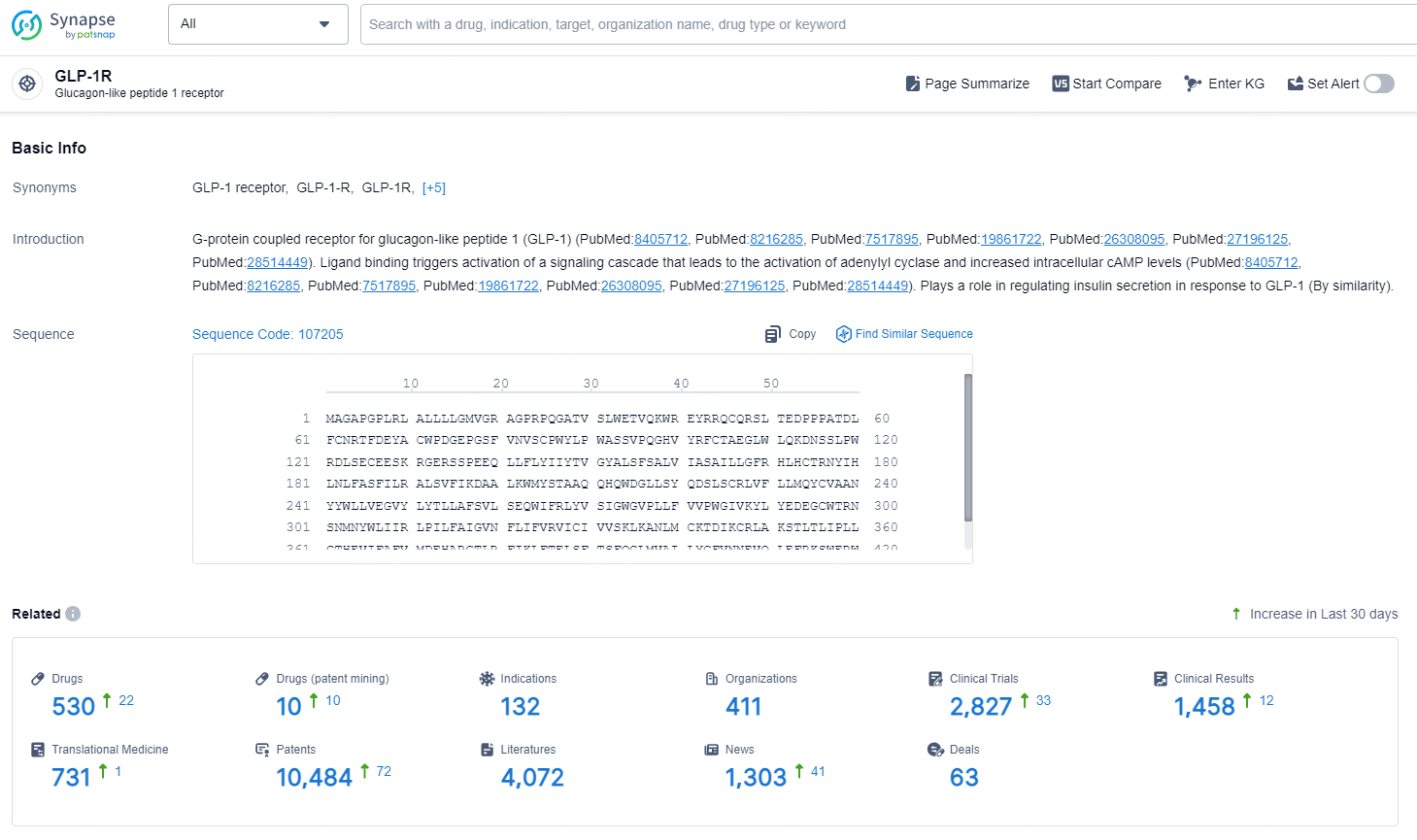
According to the data provided by the Synapse Database, As of November 5, 2024, there are 530 investigational drugs for the GLP-1R target, including 10 indications, 132 R&D institutions involved, with related clinical trials reaching 411, and as many as 10484 patents.
BMF-650 is a small molecule drug developed by Biomea Fusion, Inc. The drug targets GLP-1R and is primarily aimed at treating endocrinology and metabolic diseases. Its active indications include diabetes mellitus and obesity. Currently, BMF-650 is in the preclinical stage, which is the highest phase of development on a global scale.