Nuvalent Begins Dosing First Patient in HEROEX-1 Phase 1a/1b Trial for New HER2 Inhibitor NVL-330
Nuvalent, Inc., an early-stage biopharmaceutical firm dedicated to developing precise treatments for well-defined kinase targets in oncology, reported the launch of HEROEX-1, its Phase 1a/1b clinical study testing the new HER2-selective inhibitor NVL-330 in patients with HER2-modified non-small cell lung cancer who have undergone previous treatments.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"HER2 alterations represent a critical class of oncogenic drivers in NSCLC, encompassing both HER2 amplification and HER2 mutations, with exon 20 mutations being the most common. Despite the development of HER2-targeted therapies, no approved TKIs currently exist for patients with HER2-mutant NSCLC," noted Christopher Turner, M.D., Chief Medical Officer of Nuvalent.
"At the inception of our program, physician-scientists emphasized the requirement for a HER2 therapy that maintained efficacy against HER2 exon 20 mutations, was HER2-selective over wild-type EGFR to minimize gastrointestinal and dermatological toxicities associated with EGFR inhibition, and could penetrate the brain to manage and reduce brain metastases. The preclinical profile of NVL-330 has shown the potential to be unique by integrating these essential characteristics, thereby justifying its initial clinical trial in our HEROEX-1 study for patients with HER2-altered NSCLC," added Christopher Turner.
HEROEX-1 is a Phase 1a/1b, multicenter, open-label, dose-escalation and expansion study assessing NVL-330 in pre-treated patients with advanced HER2-altered NSCLC, including those with HER2 exon 20 mutations. The study's primary goals are to evaluate the overall safety and tolerability of NVL-330. Additional objectives include establishing the recommended Phase 2 dose, characterizing the pharmacokinetic profile, and preliminarily assessing anti-tumor activity.
"The commencement of this trial is a notable milestone for Nuvalent, marking the third program from our innovative pipeline to enter clinical development in just under three years," stated James Porter, Ph.D., Chief Executive Officer of Nuvalent. "This swift progress highlights our team's dedication to rapid development and expansion across our pipeline and our steadfast commitment to delivering precisely targeted therapies to cancer patients."
NVL-330 is a novel HER2-selective tyrosine kinase inhibitor designed to meet the combined clinical needs of treating HER2-altered tumors, including those with HER2 exon 20 insertion mutations, minimizing off-target effects from wild-type EGFR inhibition, and treating brain metastases. NVL-330 is currently being investigated in the HEROEX-1 Phase 1a/1b clinical trial for pre-treated patients with advanced HER2-altered NSCLC.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
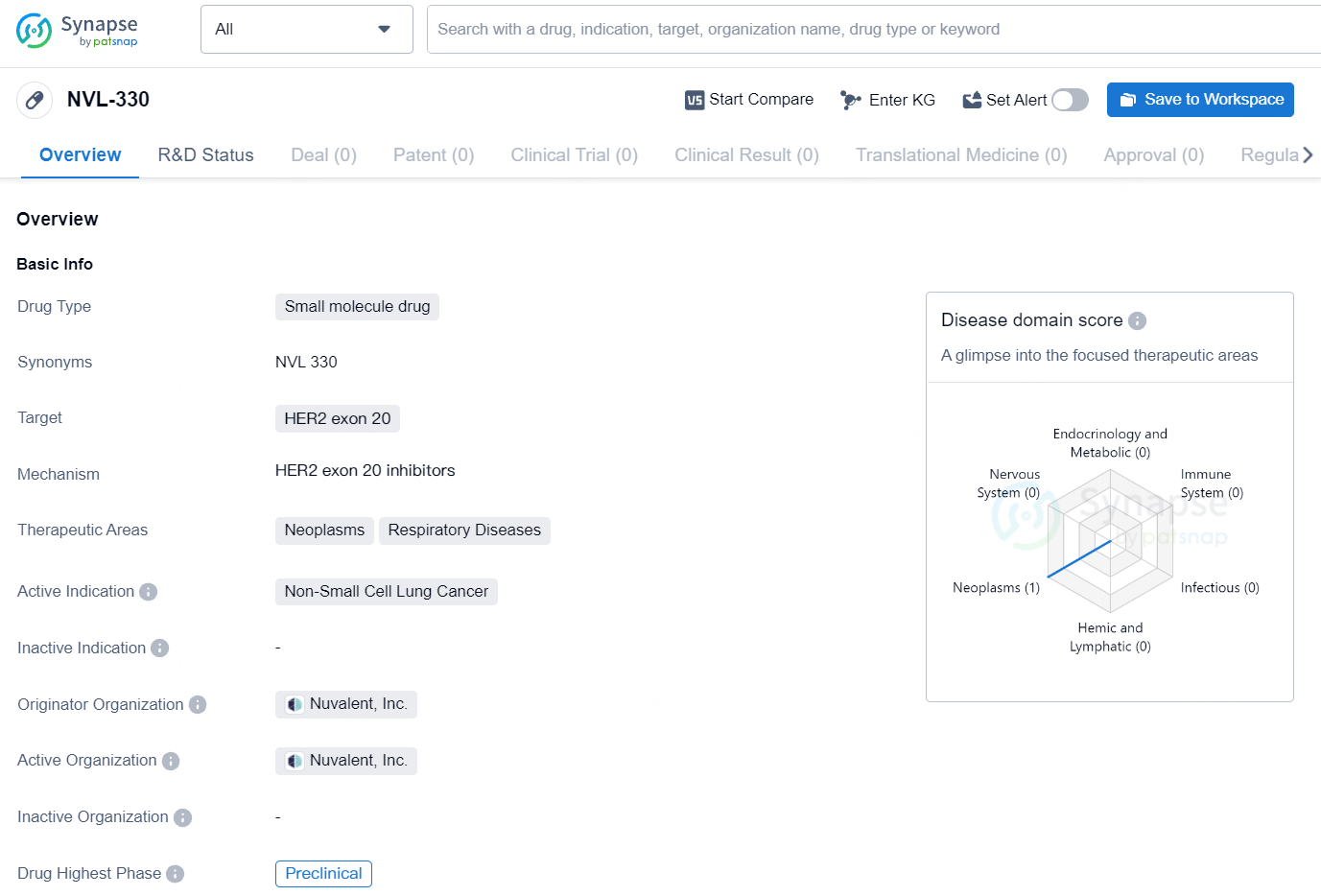
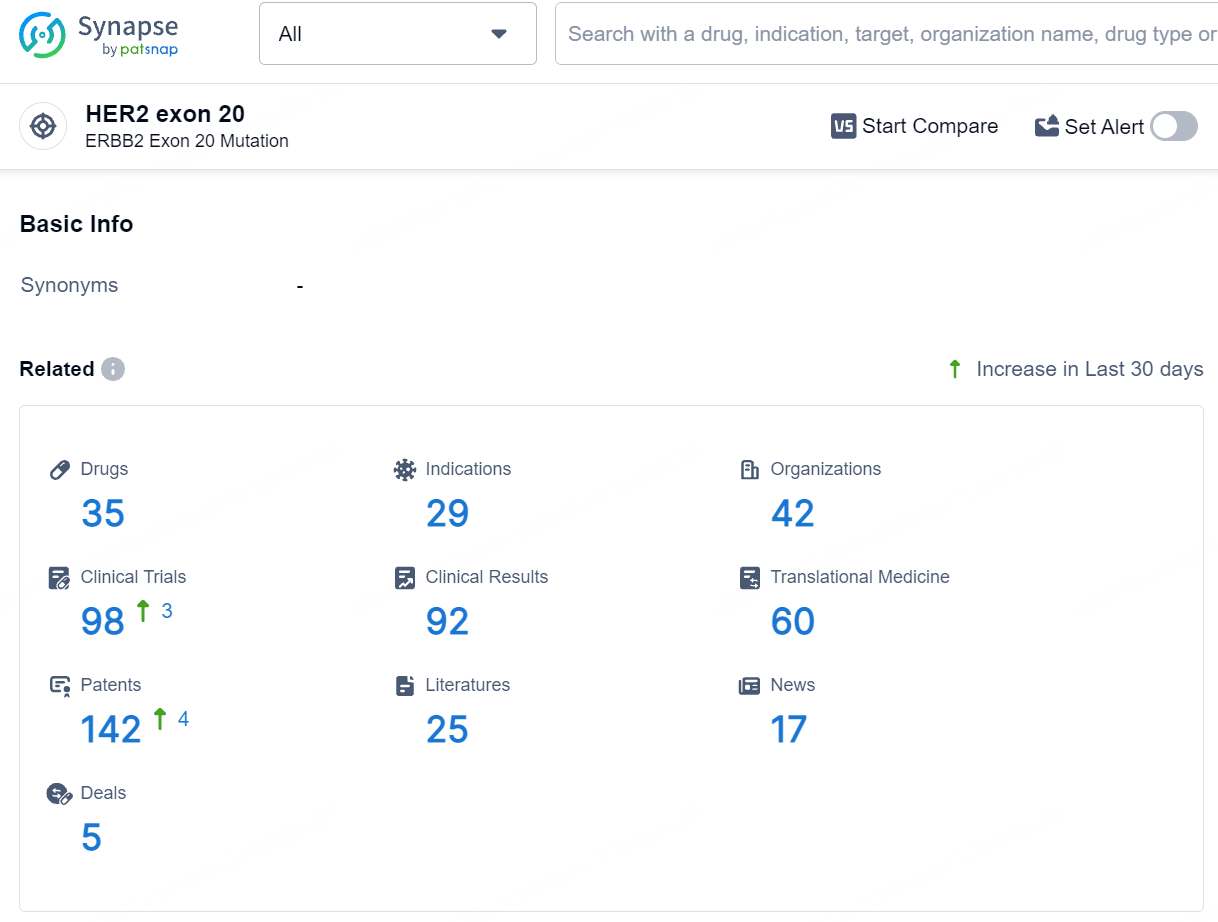
According to the data provided by the Synapse Database, As of July 24, 2024, there are 35 investigational drugs for the HER2 exon 20 target, including 29 indications, 42 R&D institutions involved, with related clinical trials reaching 98, and as many as 142 patents.
The drug NVL-330 is classified as a small molecule drug and targets the HER2 exon 20.
NVL-330 offers a glimpse into a promising drug candidate that seeks to address the challenges associated with non-small cell lung cancer and related therapeutic areas. As it continues to progress through the development pipeline, further insights into its efficacy and potential impact on patient outcomes are anticipated.