Ocugen Announces Positive DSMB Review and Begins Recruiting for Mid-Dose OCU410 Trial in Early Geographic Atrophy Study
Ocugen, Inc., which specializes in the research and development of innovative gene and cell treatments as well as vaccines, has publicized the latest proceedings from the Data and Safety Monitoring Board associated with the ArMaDa Phase 1/2 clinical trial concerning OCU410. The board has recently gathered and provided authorization to continue with the administration of the intermediary dosage level of OCU410 during the dose-intensification segment of the investigation.
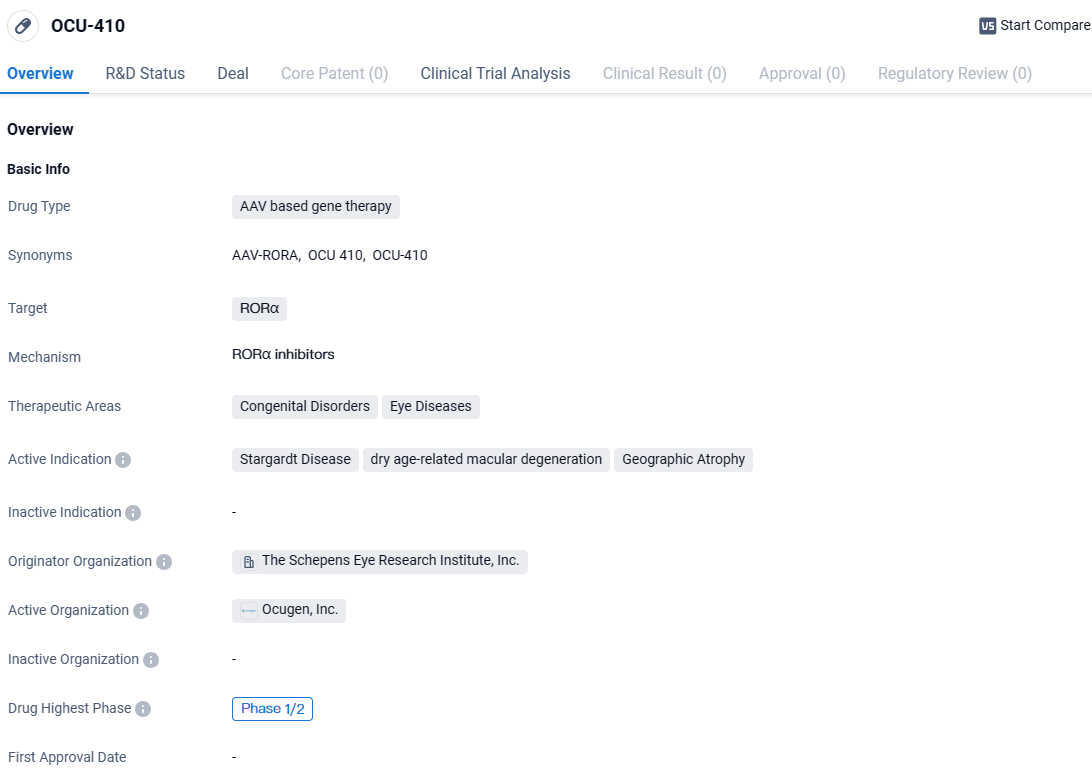
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Dr. Peter Chang, MD, FACS, who chairs the DSMB for the clinical study OCU410, has expressed the board's endorsement to escalate the dosage to the middle level for GA patient treatment," Dr. Chang reports. "Thus far, no serious unwanted effects tied to OCU410 have surfaced. It's my conviction that this decision signifies an essential advancement in pinpointing the ideal dosage strategy and a pivotal moment in OCU410's clinical evolution."
Huma Qamar, M.D., MPH, the principal medical executive of Ocugen, commented, "The encouraging feedback from the DSMB concerning the pioneering modifier gene therapy for GA serves to reinforce the previously demonstrated safety and tolerability of OCU410. There's a high level of excitement surrounding the prospects for OCU410, which promises to be a transformative, once-only treatment option, applied through a sub-retinal injection."
Current therapies that have gained approval for managing GA come with notable drawbacks; they necessitate a series of injections annually and focus on one singular aspect that contributes to GA. In contrast, OCU410 exerts its influence on several key pathways linked with the ailment, such as lipid metabolism, inflammation, oxidative stress, and the membrane attack complex.
Ocugen has dedicated itself to the pursuit of breakthrough treatments for hereditary retinal disorders and various forms of blindness, which affect a multitude of individuals globally. In the United States, GA is a severe version of the dry type of age-related macular degeneration and influences the lives of around a million individuals.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 8, 2024, there are 5 investigational drugs for the RORα target, including 8 indications, 4 R&D institutions involved, with related clinical trials reaching 3, and as many as 574 patents.
The development of OCU-410 as a gene therapy drug holds promise for the treatment of these debilitating eye diseases. By targeting RORα, the drug aims to address the underlying genetic factors contributing to the development and progression of Stargardt Disease, dry age-related macular degeneration, and Geographic Atrophy.