Adcentrx to Present Early Nectin-4 ADC Data at 2024 AACR Convention
Adcentrx, an innovator in the biotech sector focused on advancing the field of Antibody-Drug Conjugate treatments for cancer and severe health conditions, has declared its plans to unveil preclinical findings related to ADRX-0706 during the 2024 American Association for Cancer Research Annual Meeting. This prestigious event is scheduled from April 5th to 10th, 2024, in San Diego, California.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Adcentrx is poised to feature key preclinical results for its principal Antibody-Drug Conjugate (ADC) endeavor, ADRX-0706. This ADC is constructed with a cutting-edge fully human IgG1 antibody which directs itself against human Nectin-4. The antibody is bonded with a contemporary tubulin inhibitor named AP052. This linkage is achieved via Adcentrx’s own i-Conjugation™ method, which employs a cleavable linker alongside a robust conjugative chemical process.
This pioneering approach brings forth ADCs of remarkable stability that possess an impressive drug-to-antibody ratio of eight. This marks a considerable enhancement within the therapeutic range of auristatin-based ADCs, setting a new bar above the established vedotin technologies. Preclinical studies of these presentations indicate that ADRX-0706 offers an extended therapeutic window. It presents a magnified bystander impact and a superior mechanism for channeling the payload directly to Nectin-4 positive tumor cells, reducing the impact on healthy tissue.
The ADC candidate ADRX-0706 originated entirely within the research teams of Adcentrx. Its antibody constituent zeroes in on Nectin-4, a cellular adhesion protein which is abnormally present in diverse human malignancies and correlates with an adverse prognosis.
In preclinical analysis, ADRX-0706 has shown promising pharmacokinetics and safety profiles. Furthermore, it has exhibited remarkable anti-tumor activity both in cell culture experiments and in animal studies. At present, ADRX-0706 proceeds through the early stages of clinical scrutiny, specifically in a Phase 1a/b trial aimed at establishing its clinical efficacy and safety.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
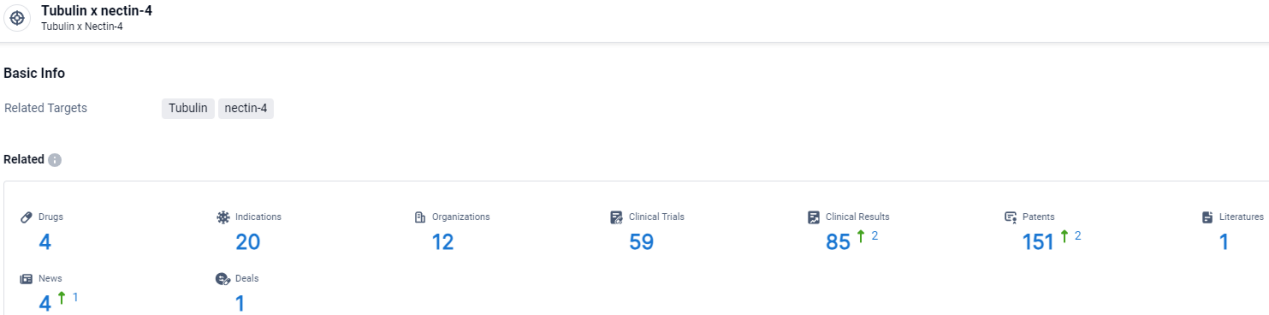
According to the data provided by the Synapse Database, As of April 3, 2024, there are 4 investigational drugs for the tubulin and nectin-4 target, including 20 indications, 12 R&D institutions involved, with related clinical trials reaching 59, and as many as 151 patents.
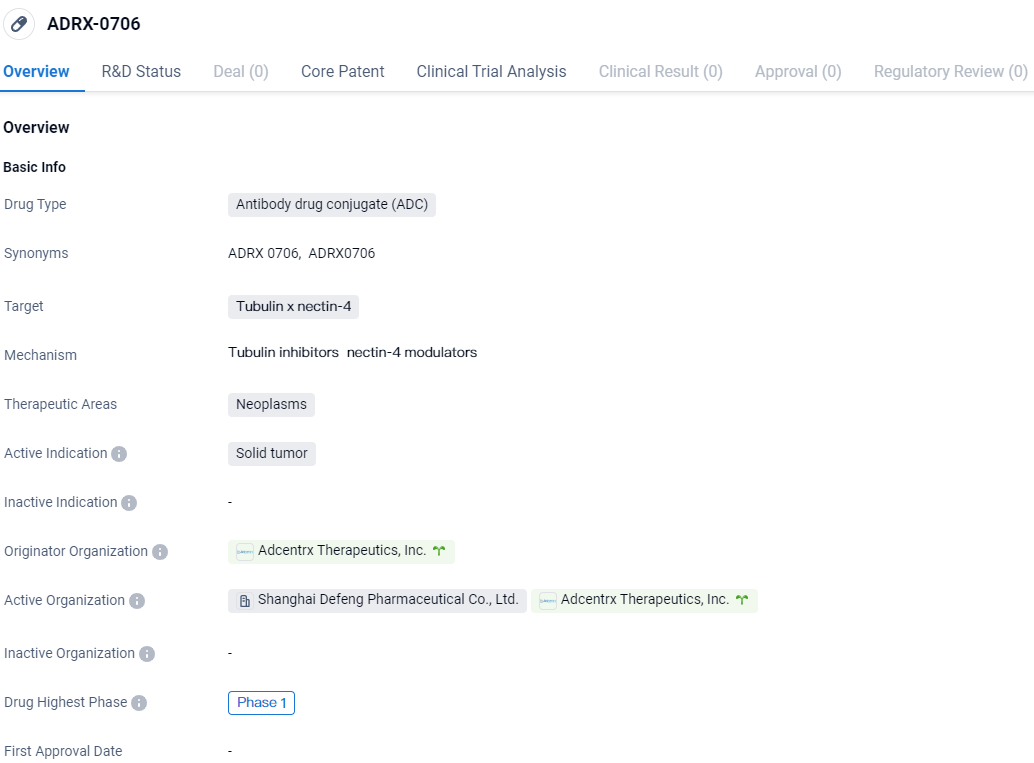
ADRX-0706 is an antibody drug conjugate that targets tubulin and nectin-4 for the treatment of neoplasms, specifically solid tumors. It is currently in Phase 1 of development on a global scale and in the IND application phase in China.