Oncternal Therapeutics updates on its phase 1/2 clinical trial for ONCT-808, a ROR1-targeted CAR T therapy for recurrent or refractory aggressive B-cell lymphoma
Oncternal Therapeutics, Inc., a biopharmaceutical firm engaged in clinical trials, recently provided an update on their Phase 1/2 study identified as ONCT-808-101. This research is progressing through the dose escalation and dose expansion stages. It is centered around assessing the therapeutic effects of the company's proprietary ROR1-targeting CAR T cell therapy, ONCT-808, expressly designed for individuals diagnosed with aggressive B-cell lymphoma that has not responded to initial treatment or has recurred. This includes subjects who have not achieved success with prior CD19 CAR T therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Beginning with an initial administration of 1x10^6 CAR T cells for each kilogram of patient weight, two out of the trio observed attained a fully realized metabolic recovery, while the remaining participant exhibited a lesser, though notable, improvement discernible through FDG PET-CT imaging. Frequent side effects noted within this particular dosage group included reductions in blood cell populations, instances of pneumonia, and a mild to moderate severity of cytokine release syndrome, as determined by the collected data up to the 4th of December, 2023.
The pioneering individual to undergo treatment at the augmented intensity of 3x10^6 CAR T cells per kilogram was an octogenarian bearing a substantial tumor load, who had formerly undergone four distinct rounds of therapeutic intervention, encompassing treatment with CD19-targeted CAR T cells. This individual succumbed to a critical adverse reaction graded at level 5, which conformed to the presentation of cytokine release syndrome (CRS) alongside a neurological condition associated with activated immune cells. Post-mortem examination did not reveal any traces of the individual's lymphoma.
The team at Oncternal has engaged in discussions and reached an understanding with the U.S. Food and Drug Administration regarding amendments to the treatment protocol that encompass alterations to the participant eligibility requirements and the consideration of reduced dosages of ONCT-808 for individuals participating in future research phases.
Dr. Salim Yazji, occupying the role of Chief Medical Officer at Oncternal Therapeutics, expressed his perspective: "Ensuring the welfare of participants in our research endeavors is a paramount concern for our team. The preliminary responses to the disease observed suggest that the ONCT-808 construct represents a potent therapeutic candidate, custom-tailored for autologous CAR T cell production, that has the potential to fulfill the urgent therapeutic needs of patients battling aggressive forms of B-cell malignancy. Faced with a clearly demarcated trajectory, we are poised to effectuate the necessary modifications to the study protocol with the greatest dispatch feasible."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
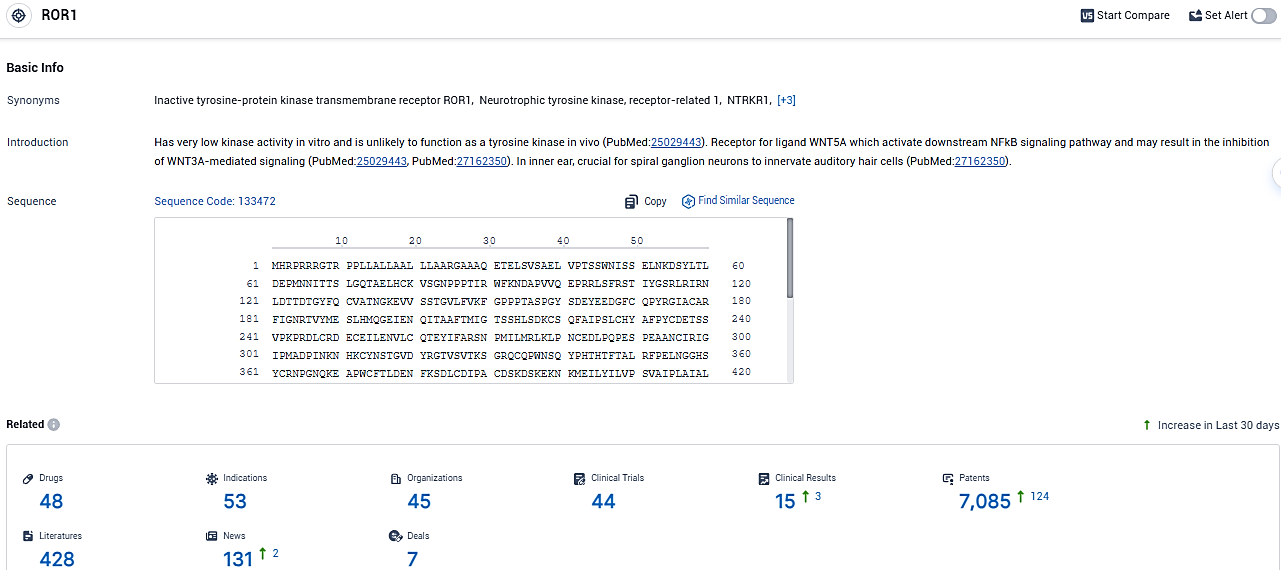
According to the data provided by the Synapse Database, As of January 04, 2024, there are 48 investigational drugs for the ROR1 target, including 53 indications, 45 R&D institutions involved, with related clinical trials reaching 44, and as many as 7085 patents.
ONCT-808 targets the ROR1 receptor. It is being investigated for its potential in treating various types of cancers, particularly those involving B-cells. The drug is currently in Phase 1/2 of development, and further research is needed to determine its safety and efficacy in larger patient population.