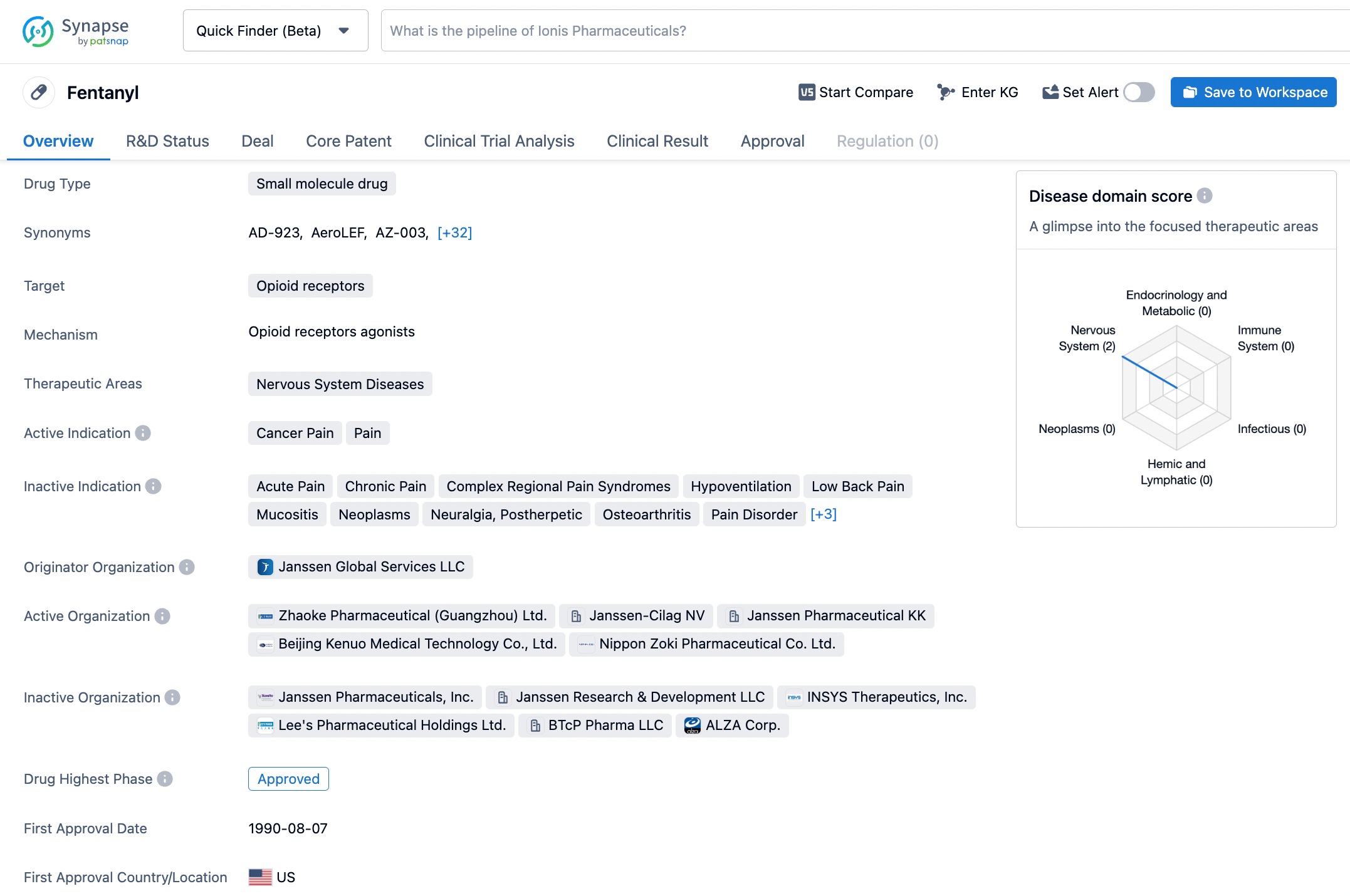
Optimize Your Synapse Experience: A Step-by-Step Guide to Searching Fentanyl
Fentanyl, a minuscule molecule drug, is classified as a COX-2 inhibitor, which selectively targets the COX-2 enzyme responsible for igniting the flames of inflammation and causing aches and agony. Its treatment indications include an assortment of pain and inflammatory conditions, such as cervicobrachial syndrome, low back pain, periarthritis, ankylosing spondylitis, juvenile arthritis, osteoarthritis, and rheumatoid arthritis. Fentanyl was sanctioned by the US FDA in January of 1995 and was formulated by C.H. Boehringer Sohn AG & Co. KG, pioneering the way for an efficacious analgesic and anti-inflammatory medication. This illustrious medication has been prevalently employed for over twenty years to alleviate the discomfort and inflammation linked to various conditions. Click on the image below to begin the exploration journey of Fentanyl through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!