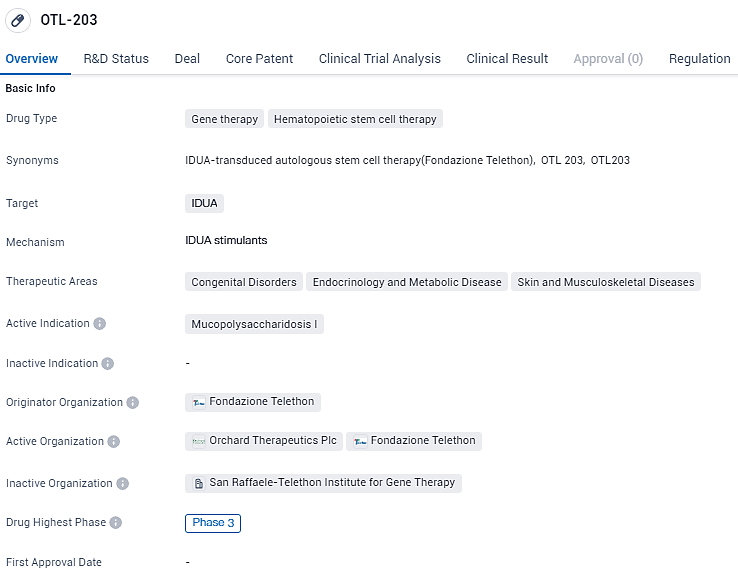
Orchard Therapeutics Begins OTL-203 Study for Hurler's Syndrome in MPS-I
Kyowa Kirin has recently integrated Orchard Therapeutics into its operations, with the strategic intent to expedite the global dispersal of pioneering gene therapy treatments. The company revealed that M Health Fairview Masonic Children’s Hospital has initiated participation of the first patient in a pivotal study assessing the impact and safety profile of the experimental hematopoietic stem cell gene treatment, OTL-203, for individuals diagnosed with Hurler syndrome, a variant of mucopolysaccharidosis type I.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The study known as HURCULES sets out to evaluate the efficacy of therapy using OTL-203 compared to the conventional approach involving allogeneic hematopoietic stem cell transplantation. The expectation is to include about 40 individuals diagnosed with MPS-IH from various locations within the United States and Europe.
MPS-I manifests as a rare genetic neurometabolic disorder, originating from a lack of the enzyme alpha-L-iduronidase within lysosomes, leading to an overaccumulation of glycosaminoglycans within several bodily systems including musculoskeletal, central nervous, cardiovascular, ocular, and auditory functionalities. The global incidence rate of MPS-I is predicted at approximately one in every 100,000 newborns.
In cases of MPS-I, a serious form referred to as MPS-IH, also recognized as Hurler syndrome, accounts for around 60 percent of diagnoses. Individuals with MPS-IH generally do not survive beyond 10 years if they do not receive treatment. The currently available treatments for MPS-IH, allogeneic hematopoietic stem cell transplant, and enzyme replacement therapy, both carry notable drawbacks.
The investigative therapy OTL-203 has garnered several regulatory endorsements, including Fast Track and Rare Pediatric Disease labels from the FDA and has been designated as a priority medicine by the European Medicines Agency. The initiative for OTL-203 was launched and initially developed through a collaboration with the San Raffaele Telethon Institute for Gene Therapy situated in Milan, Italy.
Dr. Paul Orchard, involved as a researcher in the study and positioned as professor at the University of Minnesota Medical School’s Division of Pediatric Blood and Marrow Transplantation and Cellular Therapy Program stated, “Living with MPS-IH carries substantial challenges that negatively affect patient life quality. Although stem cell transplants could offer some improvements, the procedure is not without significant risk. Therefore, novel therapies are in demand to effectively target critical symptoms of this condition, including problems with neurocognition, growth, and skeletal issues. I, along with the Orchard Therapeutics team and all global clinical collaborators, anticipate initiating this trial's recruitment process and evaluating how OTL-203 might influence the clinical trajectory of MPS-IH,” articulated Dr. Orchard.
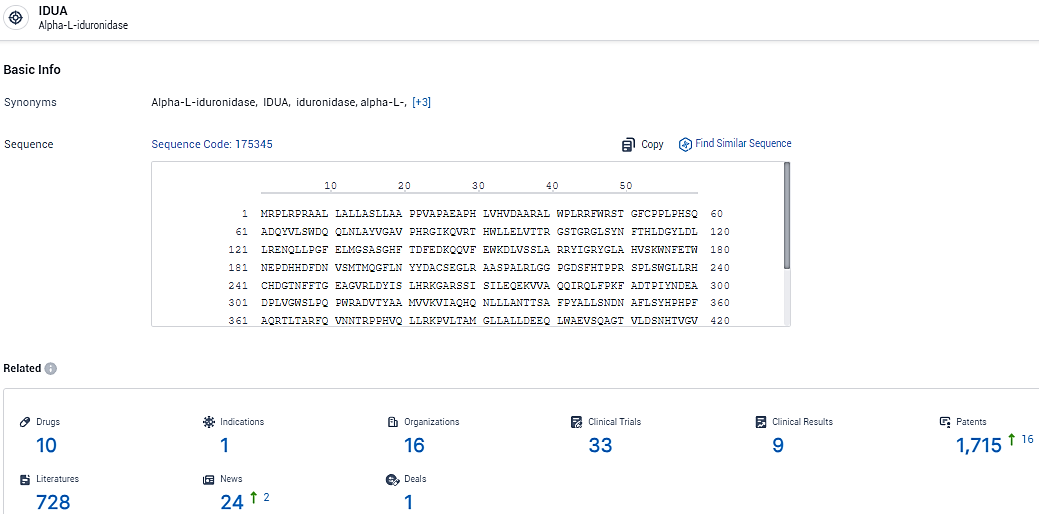
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 10 investigational drugs for the IDUA target, including 1 indications, 16 R&D institutions involved, with related clinical trials reaching 33, and as many as 1715 patents.
OTL-203 is an investigational hematopoietic stem cell gene therapy being developed for the treatment of MPS-IH. It uses a modified virus to insert a functional copy of the human IDUA gene into a patient’s cells. OTL-203 was originated by, and initially developed in partnership with, the San Raffaele Telethon Institute for Gene Therapy in Milan, Italy. OTL-203 has received Rare Pediatric Disease and Fast Track designations from the U.S. FDA, as well as priority medicines status from the EMA.