Orum Therapeutics has revealed that Bristol Myers Squibb has taken over its ORM-6151 program
Orum Therapeutics, an advanced-phase confidential biotech firm known for spearheading Dual-Precision Targeted Protein Degradation and Targeted Protein Stabilization, has declared formally that a conclusive contract has been reached, leading Bristol Myers Squibb to take ownership of Orum’s ORM-6151 scheme. ORM-6151 is a pioneering anti-CD33 antibody-stimulated GSPT1 disintegrator that has achieved approval from the FDA for Phase 1 and is intended for treating individuals with intense myeloid leukemia or hazardous myelodysplastic syndromes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Orum Therapeutics takes immense pride in partnering with Bristol Myers Squibb, renowned at an international level for their strong focus and expertise in the oncology domain and specifically, protein degradation. This partnership strengthens the value and significance of Orum's patented Dual-Precision Targeted Protein Degradation methodology, notably instrumental in widening the therapeutic window and harnessing the true power of targeted protein degraders for precision delivery to cancer cells via antibody drug conjugates," stated Dr. Sung Joo Lee, Ph.D., Orum's CEO.
"We are elated that Bristol Myers Squibb will now take over our proprietary ORM-6151 programme featuring GSPT1 degraders, the first of its kind in terms of targeted protein degraders, possessing the capability to influence and better cancer patient outcomes greatly," added Dr. Lee.
This collaboration entails Bristol Myers Squibb procuring Orum’s ORM-6151 program with an immediate payment of $100 million. Additionally, Orum Therapeutics stands to receive payments contingent on achieving specific milestones, bringing the total value of this deal to approximately $180 million. Further particulars were not shared.
Orum’s GSPT1 platform applies the company’s unique Dual-Precision Targeted Protein Degradation strategy to construct novel targeted protein degraders, synergized with the accurate antigen-specific delivery mechanisms to create ground-breaking, first-of-its-kind, tumor-selective TPDs for cancer therapy. When linked with antibodies, these payloads are planned to be administered specifically to cancer cells to degrade the intracellular target protein GSPT1 and trigger tumor cell death.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
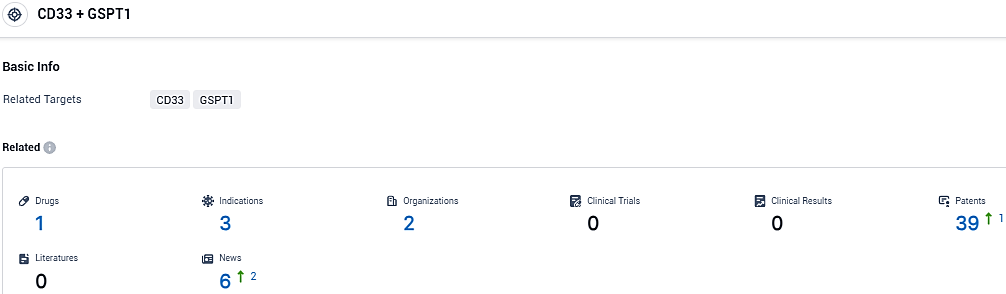
According to the data provided by the Synapse Database, As of November 16, 2023, there are 1 investigational drugs for the CD33 and GSPT1 target, including 3 indications, 2 R&D institutions involved, and as many as 39 patents.
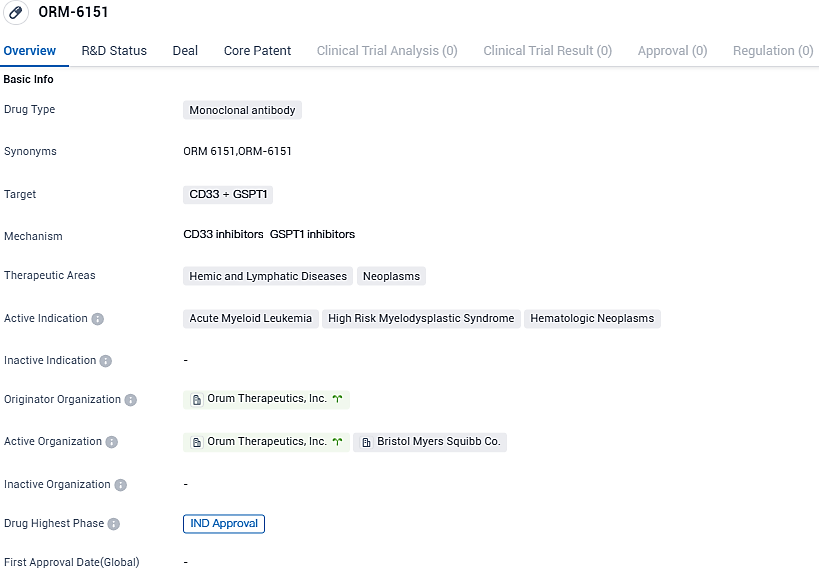
ORM-6151 targets CD33 and GSPT1 and is used in the treatment of hemic and lymphatic diseases, as well as neoplasms. The active indications for ORM-6151 include acute myeloid leukemia, high-risk myelodysplastic syndrome, and hematologic neoplasms. Orum Therapeutics is the originator organization behind the drug, and it has reached the highest phase of development with IND approval.