Overview of Exelixis’ Drug Pipeline | R&D Progress | Drug Target
Founded in 1994, Exelixis, Inc. is a biotechnology company in California focused on the anti-tumor field that has commercialized new drug products and is committed to accelerating the discovery, development and commercialization of new drugs for refractory cancer. Following early work in model systems genetics, the company established an extensive drug discovery and development platform that laid the groundwork for patients in need to work towards new cancer treatments. It currently has four marketed products, CABOMETYX® (cabozantinib), cometriq® (cabozantinib), COTELLIC® (cobimetinib) and MINNEBRO® (esaxerenone), and has partnerships with the world's leading pharmaceutical companies. Through targeted external business expansion and internal drug discovery, Exelixis continues to enrich its existing product pipeline to deliver next-generation medicines for the benefit of patients.
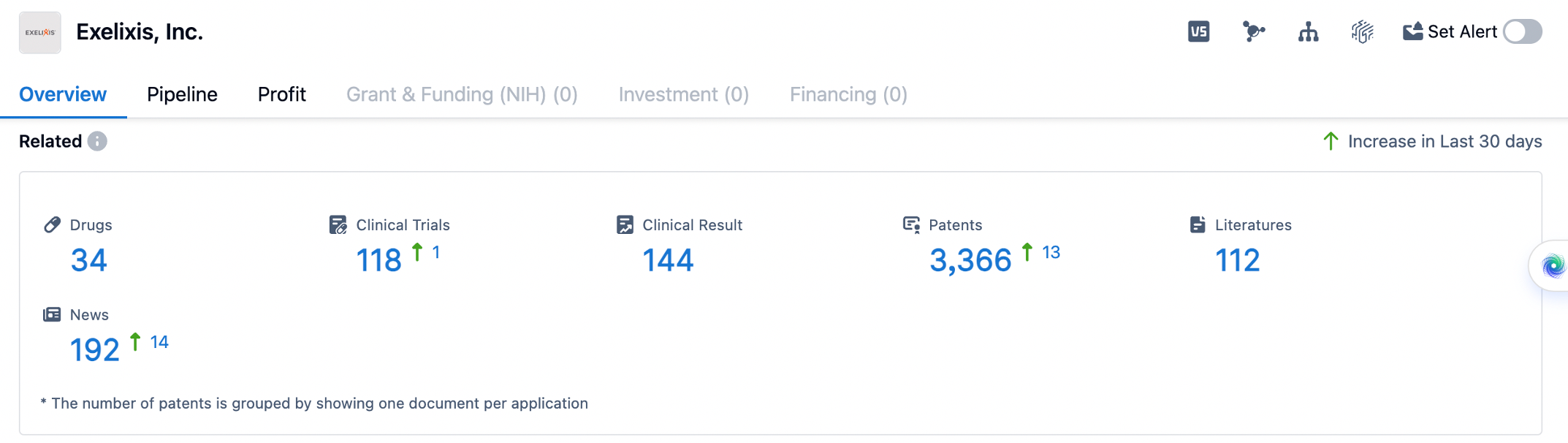
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Exelixis.
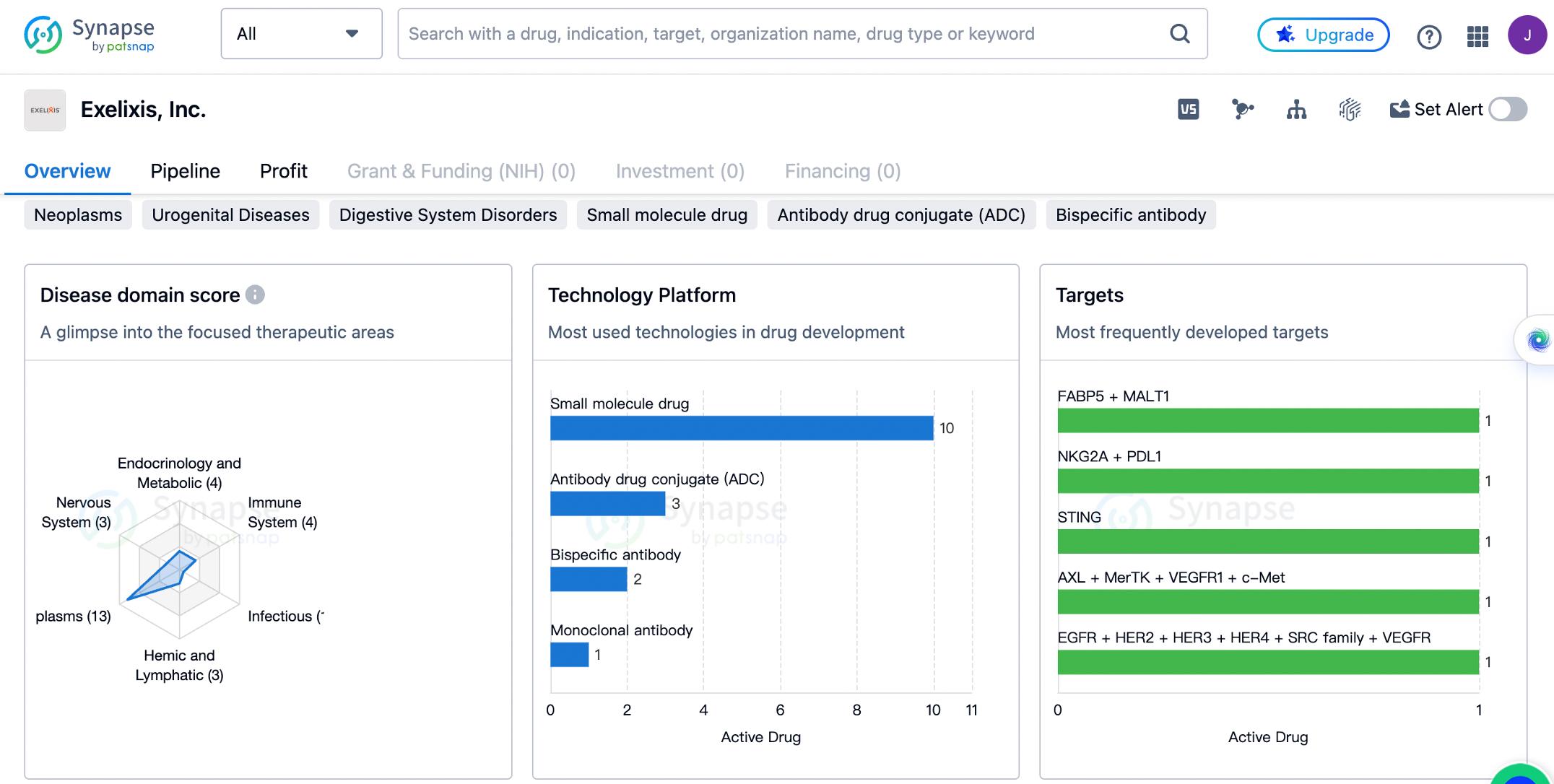
Exelixis has a diverse portfolio of drugs targeting a wide range of therapeutic areas. The company has the highest number of drugs in the field of neoplasms, with a total of 13 drugs. This indicates that Exelixis is heavily invested in developing treatments for cancer. The company also has a significant presence in the fields of skin and musculoskeletal diseases, urogenital diseases, and digestive system disorders, with 6 drugs each. This suggests that Exelixis is actively working on developing therapies for these conditions as well.
In addition to these areas, Exelixis has also developed drugs for immune system diseases, respiratory diseases, endocrinology and metabolic diseases, nervous system diseases, hemic and lymphatic diseases, mouth and tooth diseases, cardiovascular diseases, and other diseases. While the number of drugs in these areas is relatively lower compared to neoplasms and other major therapeutic areas, it indicates that Exelixis has a diverse pipeline and is exploring various disease areas.
The most frequently developed targets by exelixis.
The targets include FABP5 + MALT1, NKG2A + PDL1, STING, AXL + MerTK + VEGFR1 + c-Met, EGFR + HER2 + HER3 + HER4 + SRC family + VEGFR, CDK7, HSP90B, RORG, CD47 + PDL1, AXL, SMO, 5T4 + Tubulin, AXL + RET + ROS1 + TYRO3 + Tie-2 + TrkB + VEGFR1 + VEGFR2 + VEGFR3 + c-Kit + c-Met, MEK1 + MEK2, and Tubulin + tissue factor. These targets represent a diverse range of proteins and pathways that are implicated in various diseases. For example, EGFR, HER2, HER3, HER4, and VEGFR are all involved in signaling pathways that play a crucial role in cancer development and progression. AXL is a receptor tyrosine kinase that has been implicated in multiple cancer types and is considered a promising target for therapy. CDK7 is a cyclin-dependent kinase that regulates the cell cycle and is being explored as a target for cancer treatment. These targets highlight Exelixis' focus on developing therapies that target specific proteins or pathways involved in disease pathogenesis.
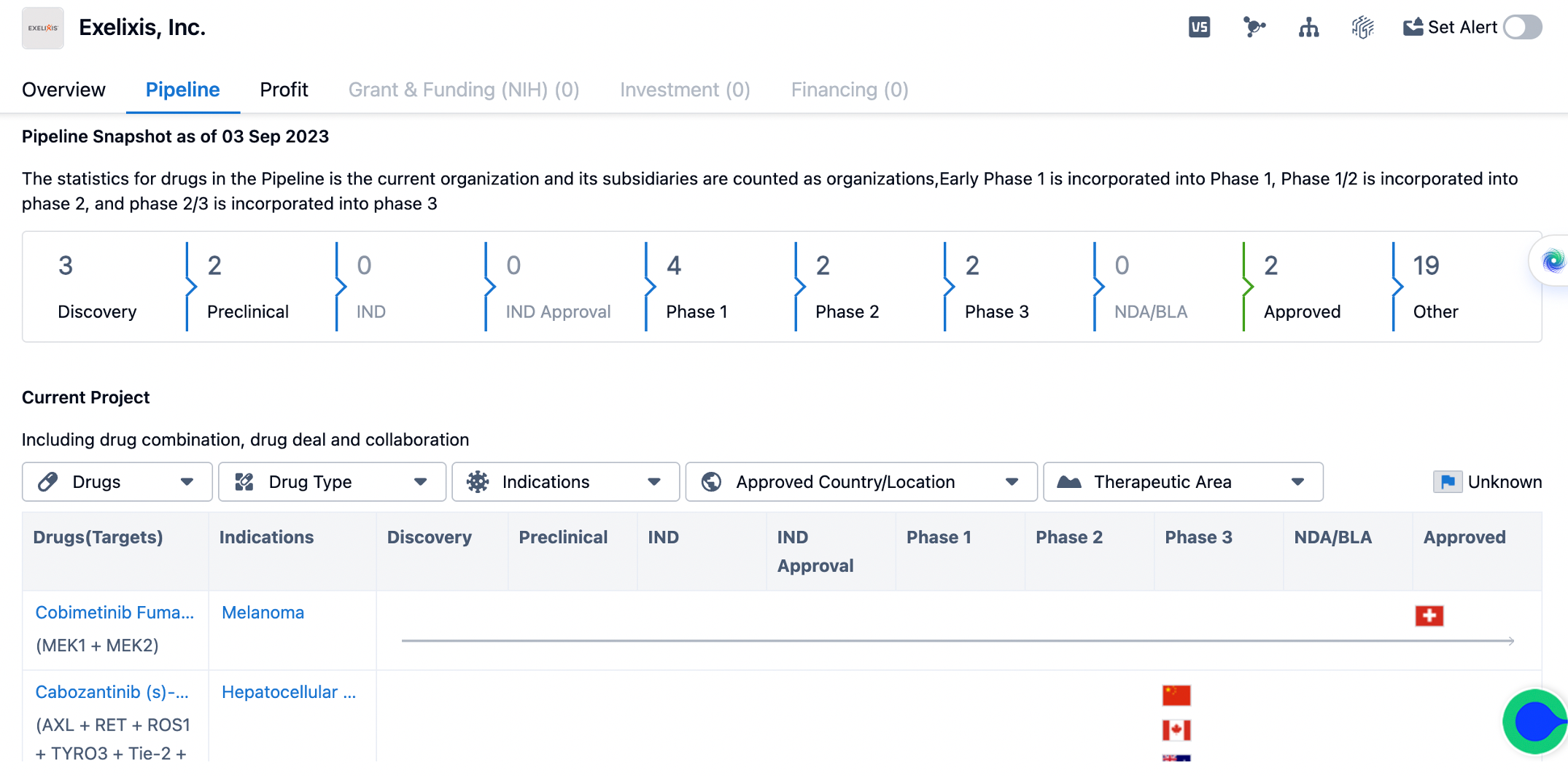
An overview on exelixis' pipeline
The pipeline includes drugs in various stages, from discovery to approved. As of the given date, Exelixis has 3 drugs in the discovery phase, indicating ongoing research and development efforts. The company also has 2 drugs in the preclinical phase, which suggests that these drugs are being tested in laboratory and animal models to assess their safety and efficacy before advancing to clinical trials. In terms of clinical development, Exelixis has 4 drugs in phase 1, 2 drugs in phase 2, and 2 drugs in phase 3. Phase 1 trials are the first stage of testing in humans and are primarily focused on assessing the safety and dosage of the drug. Phase 2 trials involve a larger number of patients and aim to evaluate the drug's effectiveness and side effects. Phase 3 trials are the final stage before seeking regulatory approval and involve a larger number of patients to further assess the drug's safety and efficacy.
Exelixis has 2 drugs that have received approval, indicating successful completion of clinical trials and regulatory review. These drugs have demonstrated their safety and efficacy and are now available for use in patients. It is worth noting that there are no drugs listed in the NDA/BLA category, which suggests that Exelixis does not have any drugs currently under review by regulatory authorities for approval. In addition to the drugs in various stages of development, Exelixis has 19 drugs under the "Other" category in the pipeline. This category likely includes drugs that are in earlier stages of development or are being explored for potential therapeutic applications. These drugs may still require further research and testing before advancing to clinical trials.
In summary, Exelixis is a biopharmaceutical company that focuses on the development and commercialization of innovative therapies, with a particular emphasis on oncology. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a significant focus on neoplasms. Exelixis is actively developing drugs targeting specific proteins and pathways implicated in disease pathogenesis. The company has a pipeline of drugs in different stages of development, from discovery to approved, indicating ongoing research and development efforts. Overall, Exelixis is dedicated to advancing the field of biomedicine and bringing new treatment options to patients.




