Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(2)
Based on the characteristics of ADC drugs, we have summarized the process for conducting patent research on ADC drugs.You can start by reading the following article to get background information.
Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(1)
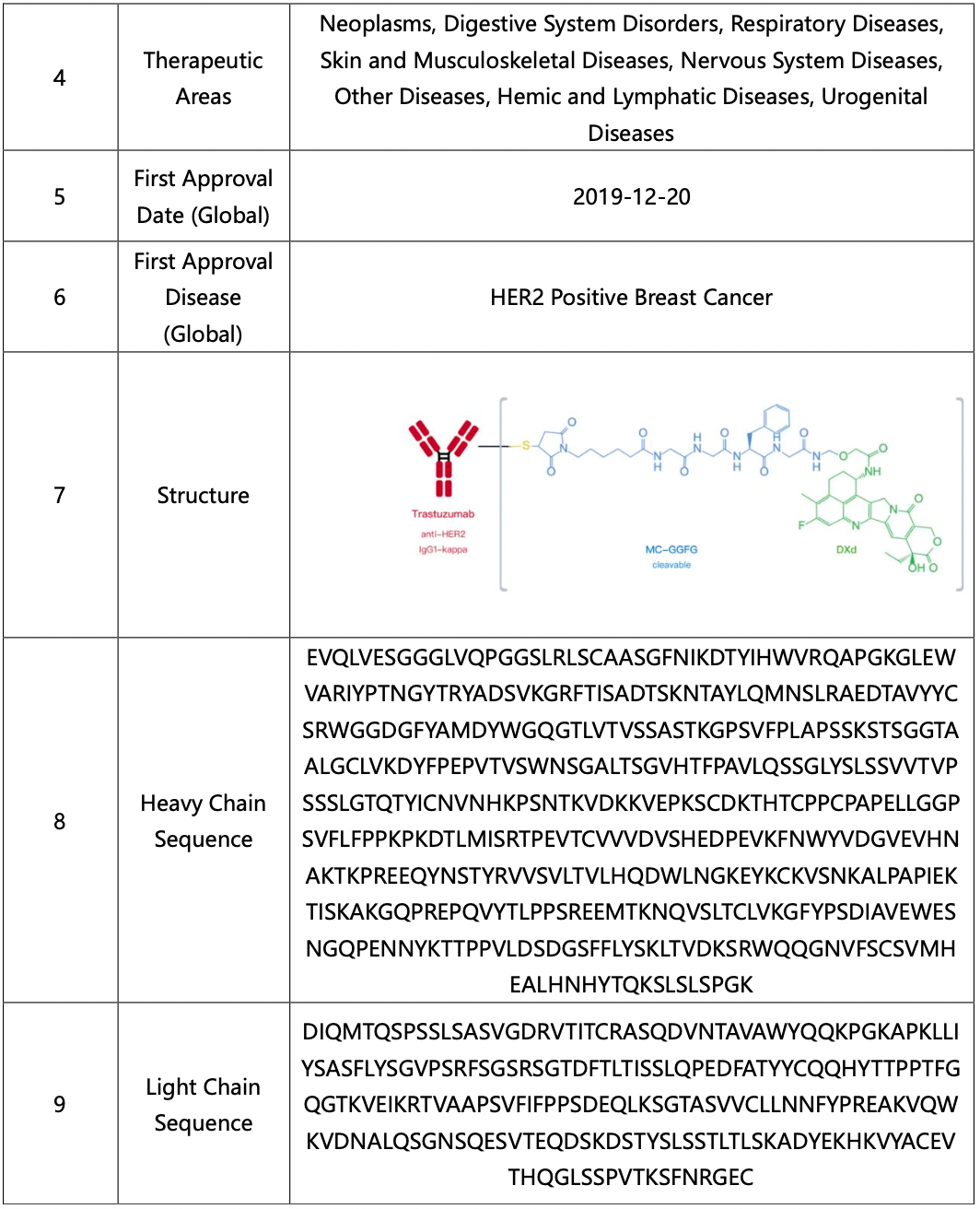
DS-8201, also known as fam-trastuzumab deruxtecan-nxki (Enhertu), was officially approved for market by the U.S. Food and Drug Administration (FDA) in December 2019 and by the China's National Medical Products Administration (NMPA) in February 2023. This novel HER2-targeting ADC is effective not only against tumors with high HER2 expression that respond to trastuzumab, but also shows good efficacy against tumors with low HER2 expression where trastuzumab is ineffective. Additionally, it can treat brain metastases from tumors and has significant clinical application value in HER2-negative breast cancer, HER2-positive breast cancer, HER2-expressing gastric adenocarcinoma, and metastatic non-small cell lung cancer.
As Daiichi Sankyo's flagship product, DS-8201's global sales in the first quarter of 2024 reached $879 million, an increase of $348 million compared to the same period in 2023. This patent research guide focuses on DS-8201 and includes the most recent data as of April 28, 2024.
Retrieval of Basic Information on DS-8201
First, log in to Patsnap Synapse and search for "DS-8201" from the homepage. Next, click on the retrieved DS-8201 drug card and navigate to the ‘Overview’ tab.
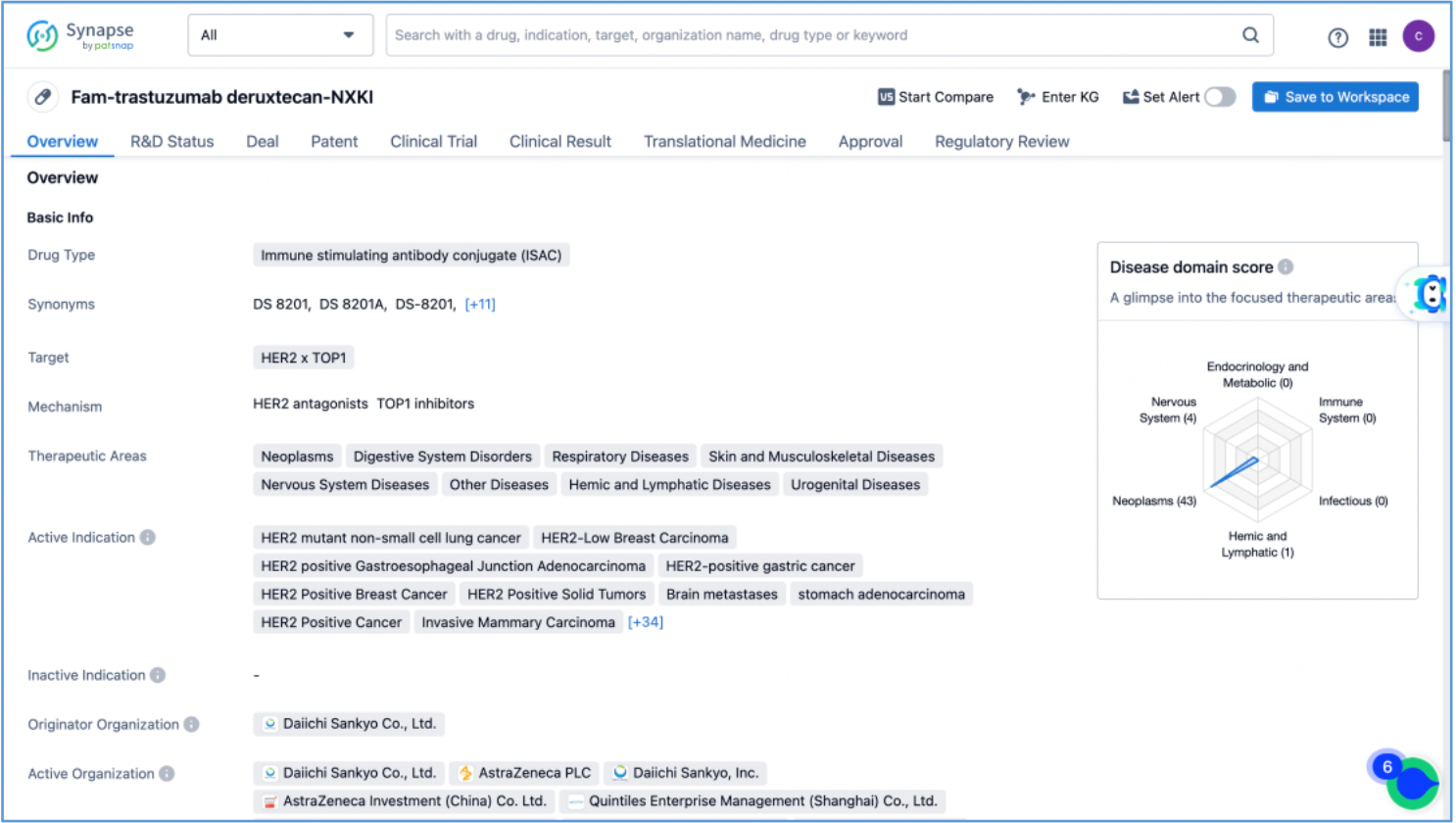
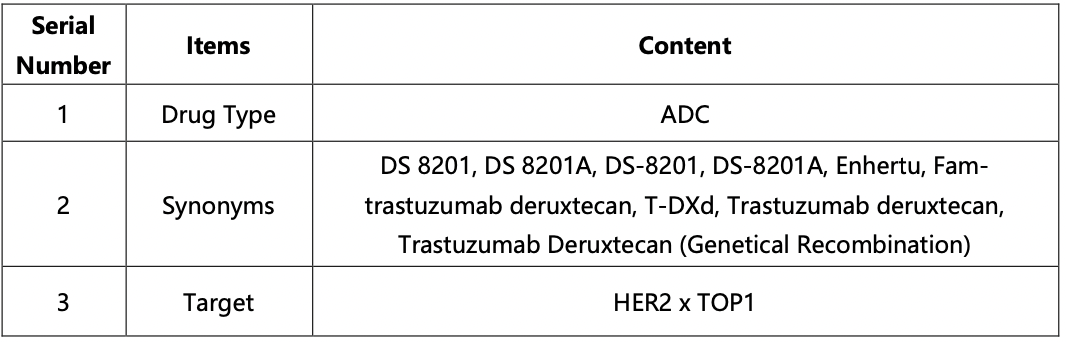
The compilation of basic information regarding DS-8201
Initial Patent Search and Core Patent Identification for DS-8201 in Patsnap Synapse
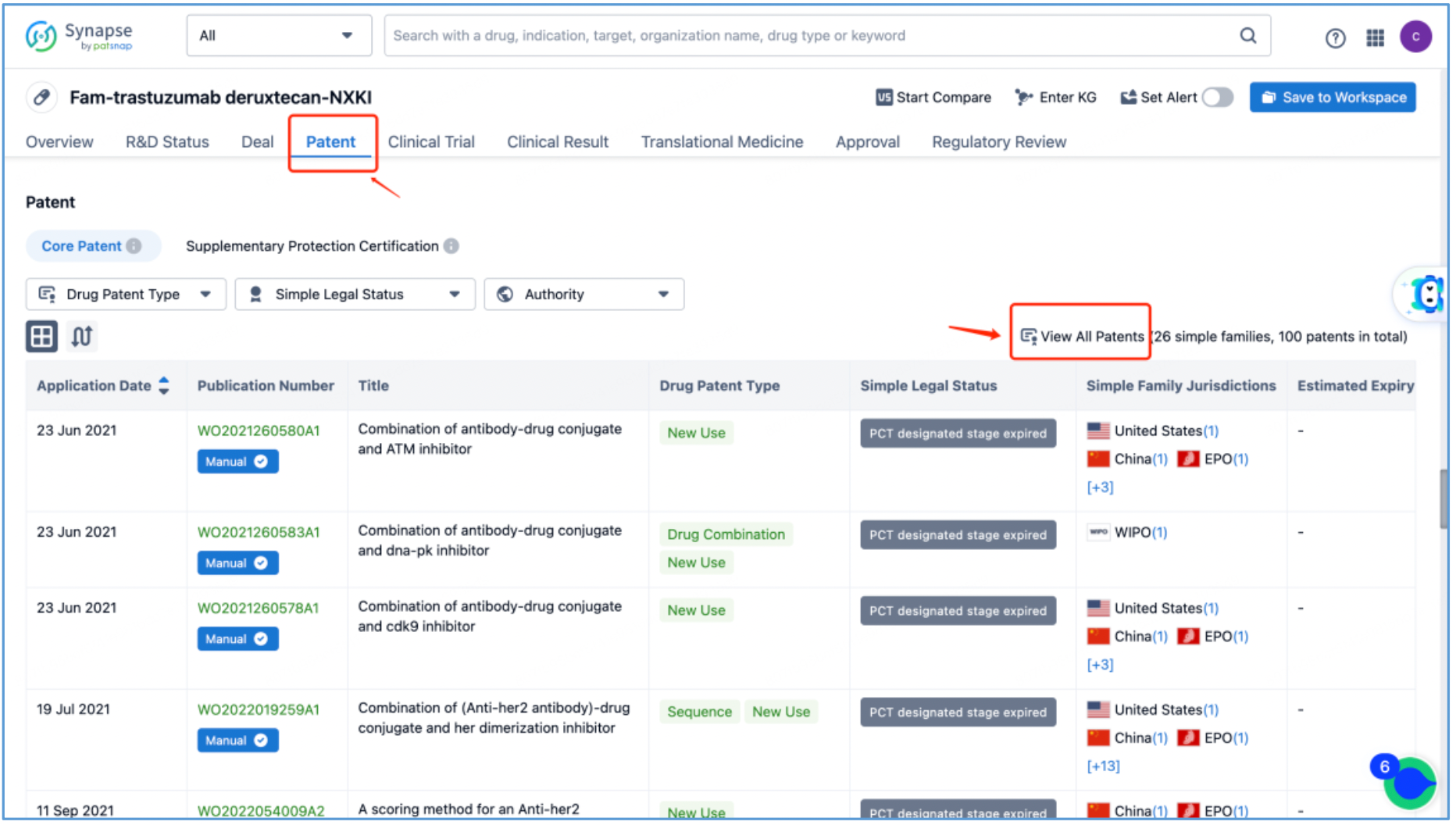
From the DS-8201 page, select the "Patent" tab and click "View All Patents" on the right to retrieve all the patents related to DS-8201.
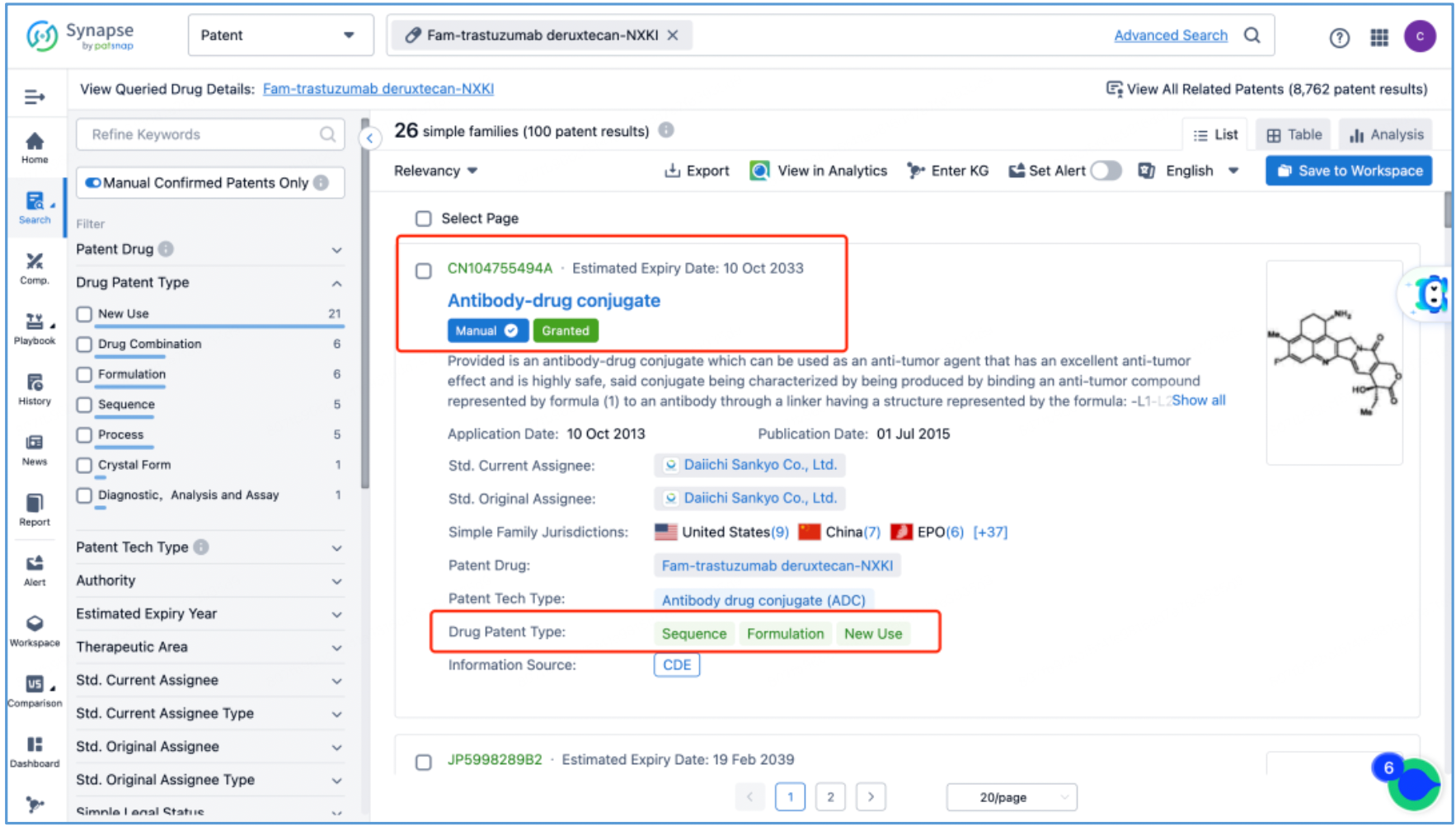
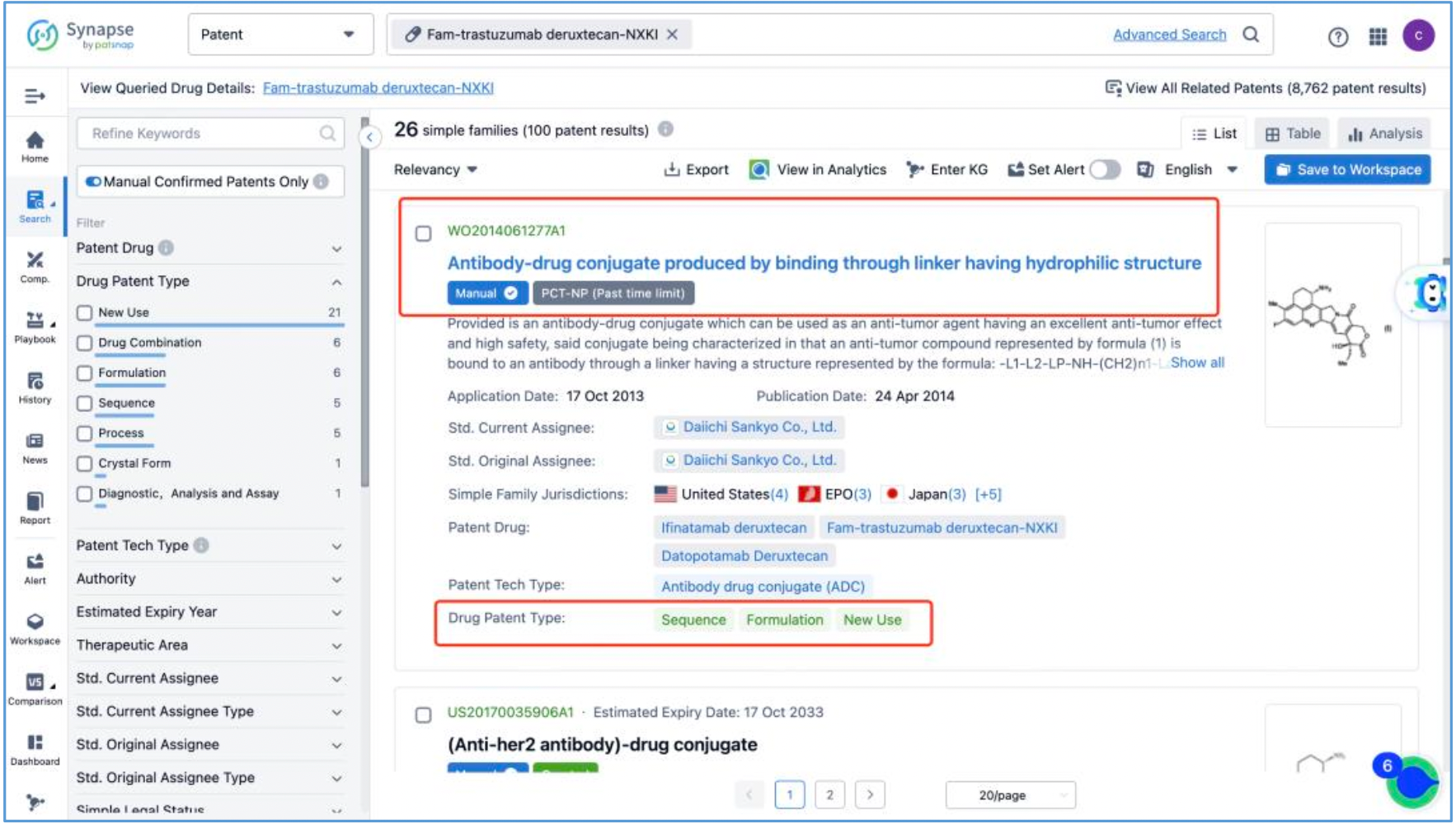
Next, select the resulting 26 simple families and add them to a new Workspace. Based on Synapse’s manual indexing, the following can be preliminarily deduced as being core product patent families: patent application CN104755494A, titled "Antibody-Drug Conjugate", patent application WO2014061277A1, titled "Antibody-drug conjugate produced by binding through linker having hydrophilic structure", patent application JP5998289B2, titled "Anti-HER2 antibody-drug conjugate", and patent application US20170035906A1, titled "(Anti-HER2 Antibody)-Drug Conjugate". The above patent families are classified as Sequence, Formulation, and New Use patent types. Thus, it is preliminarily determined that the above four patent families are core product patents. Subsequently, CN104755494A WO2014061277A1, CN105829346B (simple family patent of JP5998289B2), and US11185594B2 (authorized versions of US20170035906A1) are selected for further analysis.
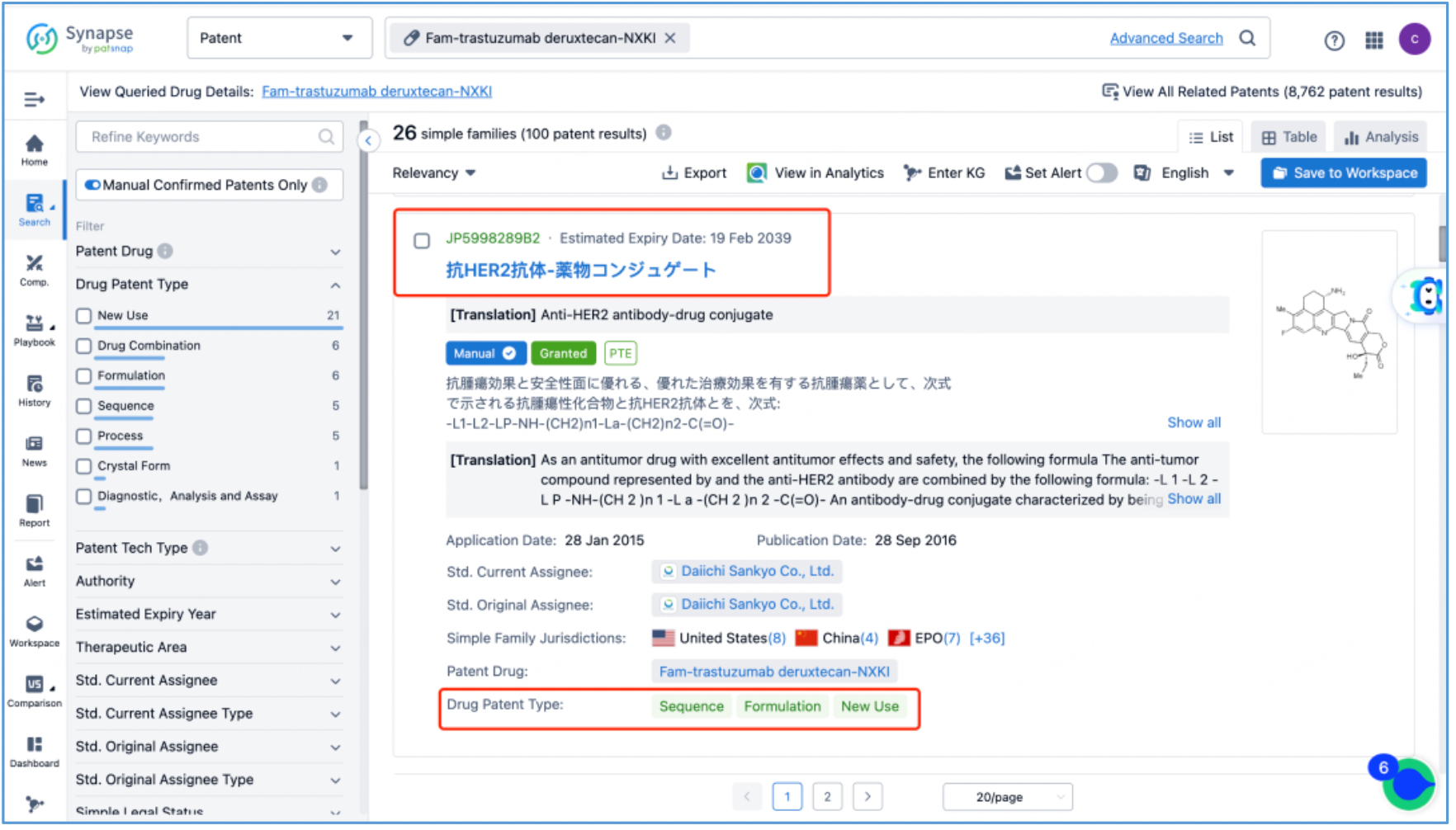
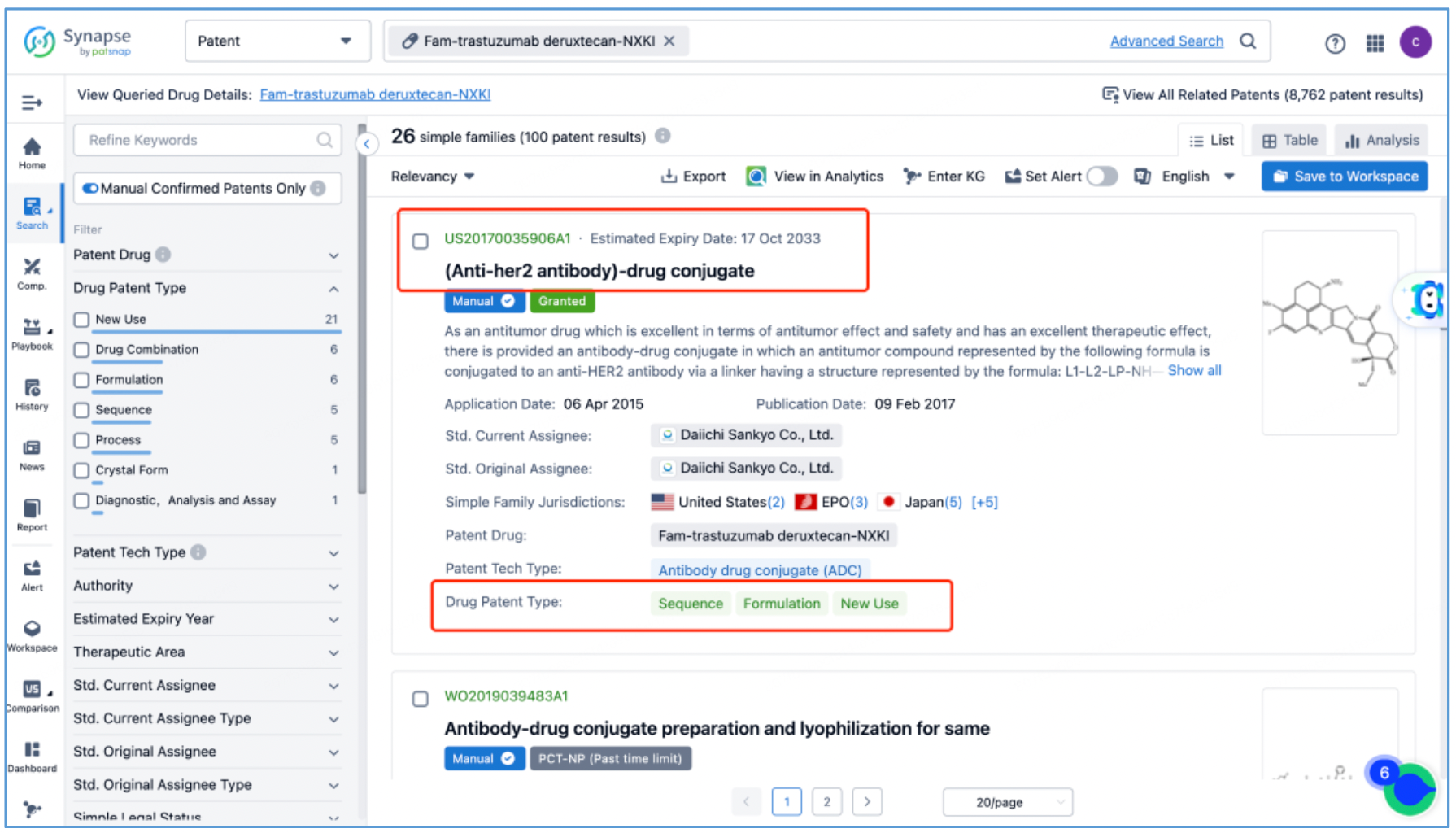
Analysis of Core Product Patents to Ascertain the Structure of DS-8201
Next, launch Patsnap Analytics by clicking on ‘View in Analytics’ to perform an in-depth analysis of each core product patent family. Then, open the patent details for CN105531288A.
Upon examination, it is initially determined that the core substance patent CN105531288A (Anti-PD1 antibodies and their use as therapeutics and diagnostics) comprises 67 simple family members and has been cited 39 times. This patent outlines antibodies that specifically target PD-1, impeding the cell signal transduction and activity mediated by PD1 in immune cells. It also defines a set of amino acid residues necessary for binding PD1 ligands and describes the application of these antibodies in treating or diagnosing cancers, infectious diseases, or other pathological conditions regulated by PD1-mediated functions.
From this assessment, it is evident that the core substance patent safeguards multiple antibody sequence CDRs. However, it does not explicitly specify the CDR sequences.
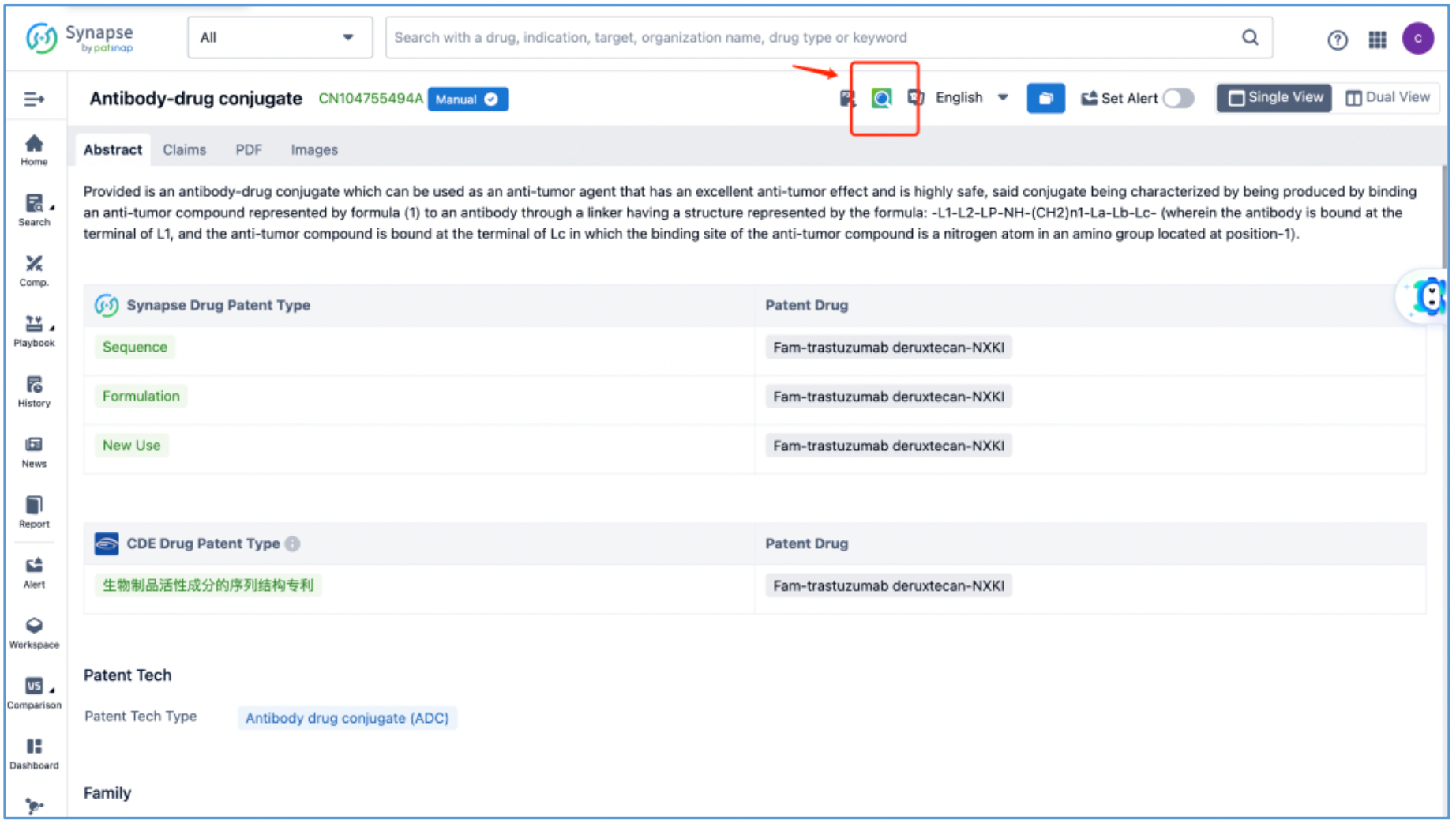
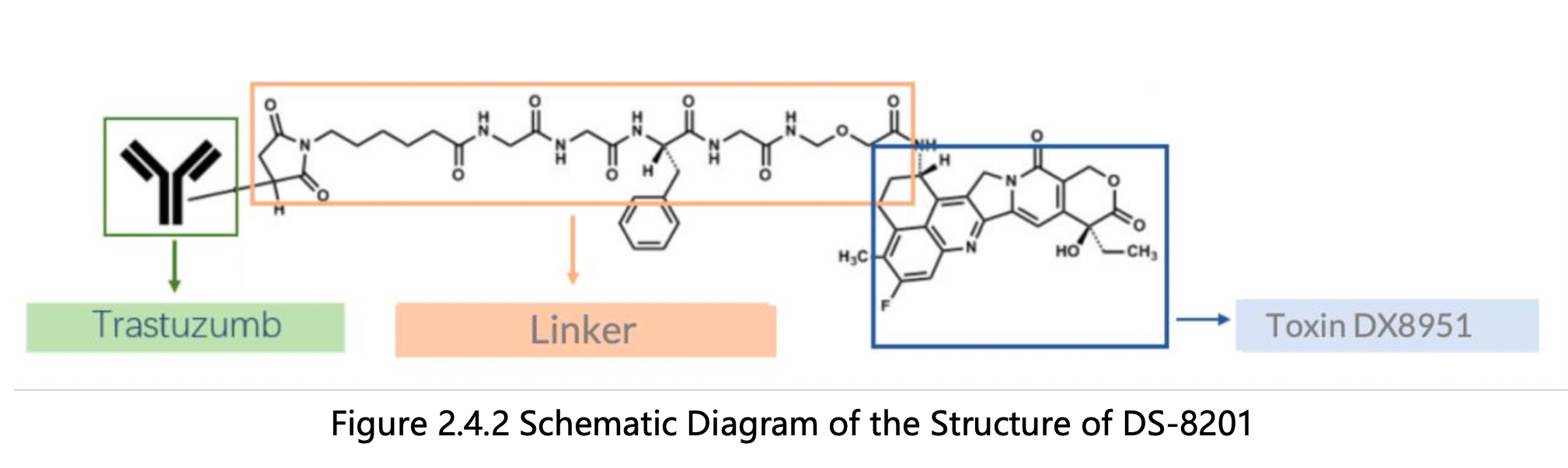
By reading the claims of CN104755494B, WO2014061277A1, CN105829346B, and US11185594B2, we find that the linker structure described in the claims of WO2014061277A1 and US11185594B2 is different from that described in the claims of CN104755494B and CN105829346B. Through cross-validation with the relevant information summarized in section 2.2 and publicly available information retrieved online, we confirm that the structures protected in the claims of CN104755494B and CN105829346B match DS-8201, as illustrated in Figure 2.4.2. The linker structure described in the claims of WO2014061277A1 and US11185594B2 corresponds to other structures in the same series. Furthermore, we find that the claims of CN105829346B include specific toxin compound structures, linker structures, and information on the antibody heavy and light chains. Therefore, CN105829346B is further analyzed as the core product patent.
The original patents for the three components of DS-8201 were not found among the 26 simple patent families. To locate the original development history of each component, the next section of this guide will analyze the original patents of each component in section 2.5 of this report through a structure and sequence search.
For more information, you can click the following article link:
Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(3)
For more information, please click the image link below to access the full report.