Revance enters the American medical market by introducing DAXXIFY® to treat cervical dystonia
Revance Therapeutics, Inc. has initiated the market release of DAXXIFY (DaxibotulinumtoxinA-lanm) for injection, aimed at treating cervical dystonia. This launch introduces a significant new therapy alternative for both physicians and patients dealing with this chronic and distressing condition. Additionally, this launch signifies Revance’s first foray into the expansive and burgeoning U.S. market for therapeutic neurotoxins.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"DAXXIFY represents a pivotal advancement for Revance, epitomizing the successful realization of our longstanding commitment to pioneering innovation in the field of therapeutics. With its unique clinical attributes, appealing value, and safety features, DAXXIFY is poised to meet the diverse needs of key groups including patients, physicians, and insurers," stated Mark J. Foley, President and CEO.
Following its approval by the FDA in August 2023 for the treatment of cervical dystonia, Revance rolled out the DAXXIFY cervical dystonia PrevU program aimed at enhancing treatment effectiveness and facilitating seamless integration into medical practices.
In anticipation of DAXXIFY's market introduction, Revance developed a robust commercial framework, secured a permanent J-Code from the U.S. Centers for Medicare & Medicaid Services, activated support for reimbursement to ease adoption hurdles, and initiated programs to help patients manage expenses.
David A. Hollander, M.D., MBA, Chief Medical Officer and Global Therapeutics Franchise Lead, emphasized, "A key focus for us has been to reduce obstacles and expand access to DAXXIFY for a substantial number of CD patients experiencing early symptom recurrence who find existing toxin therapies insufficient."
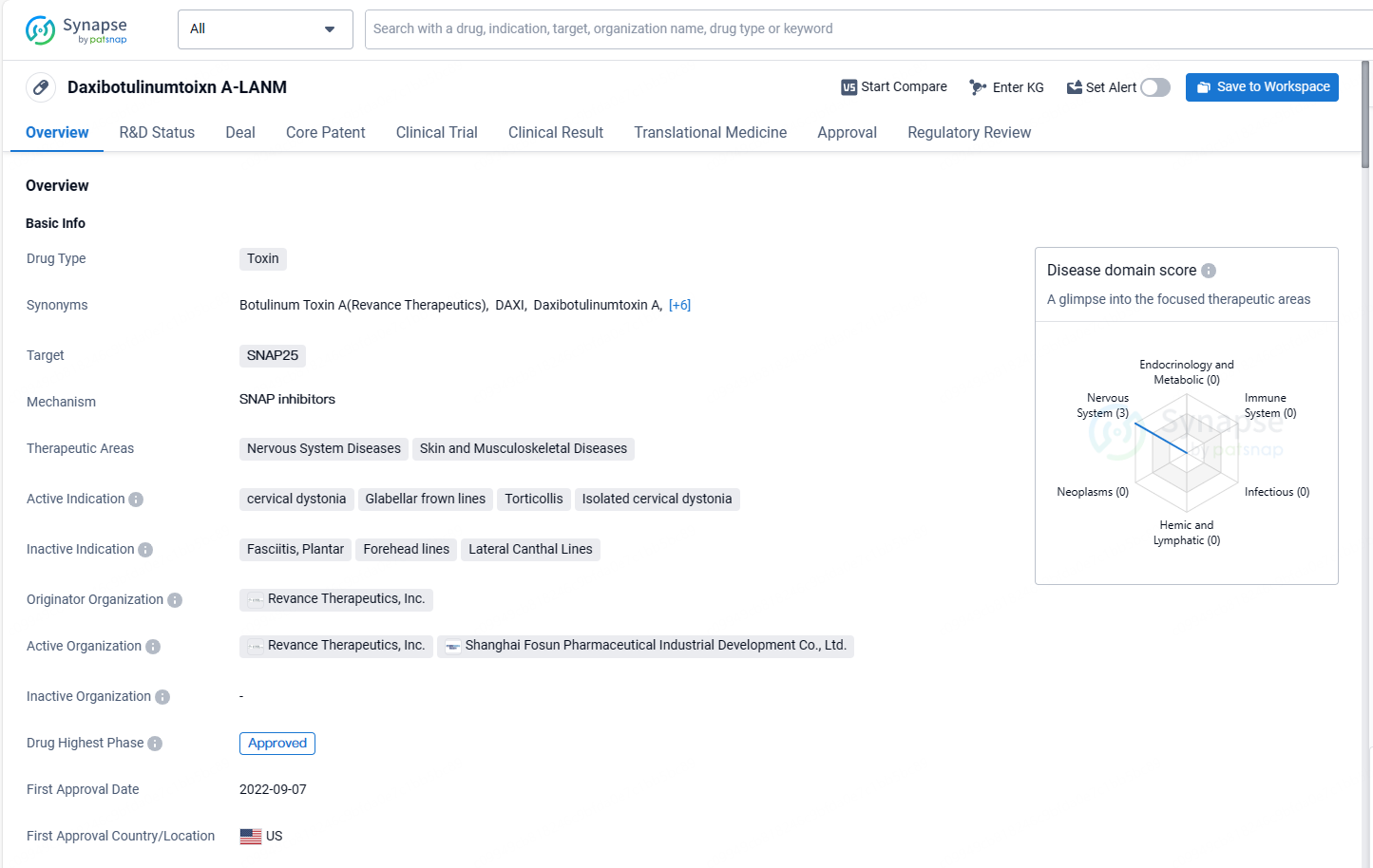
DAXXIFY® (daxibotulinumtoxinA-lanm) is an injection that inhibits acetylcholine release and acts as a neuromuscular blocker. It is approved for temporarily enhancing the look of moderate to severe glabellar lines due to corrugator and/or procerus muscle activity in adults and for treating adult cervical dystonia.
DAXXIFY® (DaxibotulinumtoxinA-lanm) is the only FDA-approved long-duration, peptide-based neuromodulator in the U.S., indicated for both improving glabellar lines and managing cervical dystonia in adults. Featuring Revance's Peptide Exchange Technology™, this innovative agent excludes human serum albumin and animal derivatives. It represents the first significant breakthrough in neuromodulator formulations in over three decades, manufactured entirely in the U.S.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
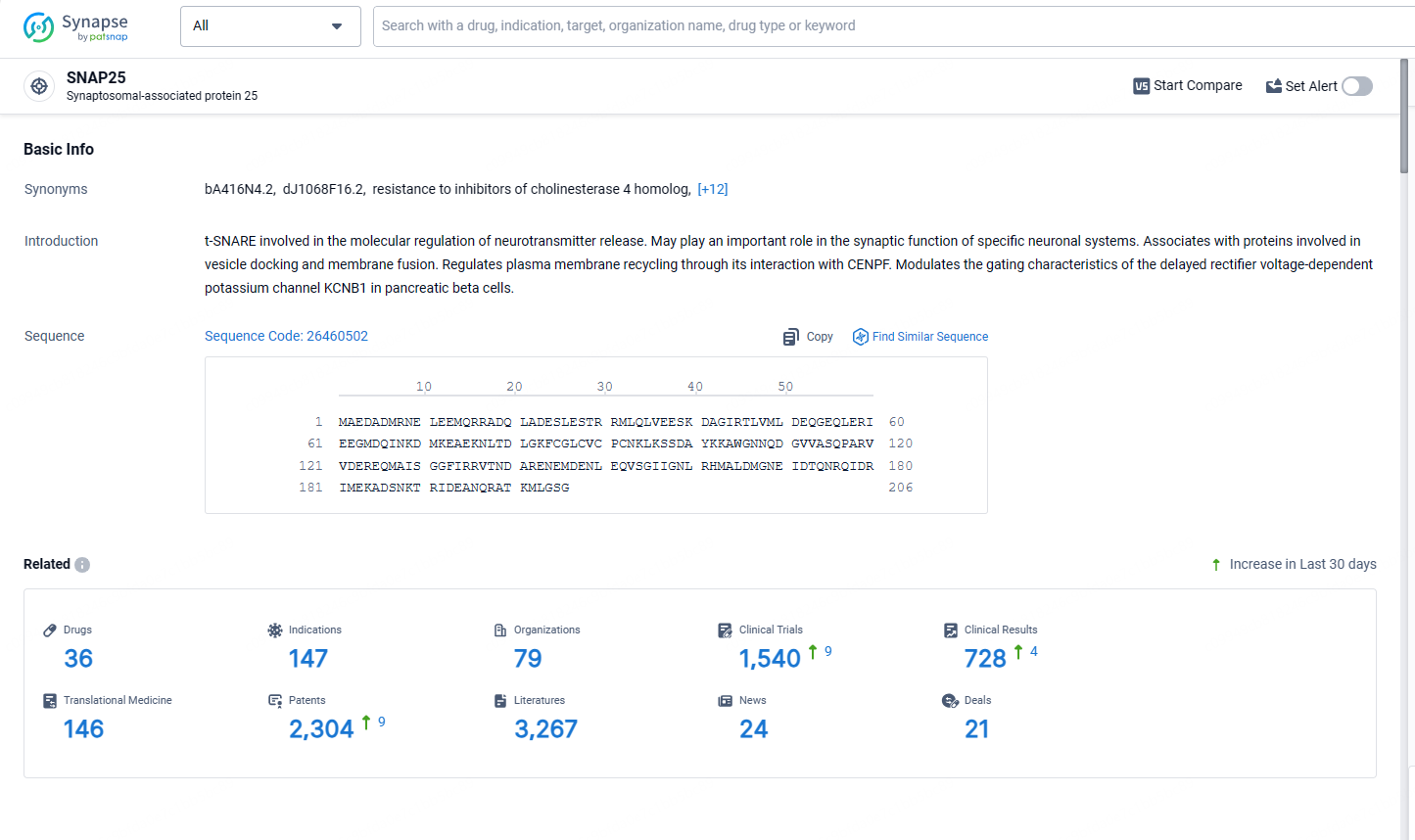
According to the data provided by the Synapse Database, As of May 13, 2024, there are 36 investigational drugs for the SNAP25 target, including 147 indications, 79 R&D institutions involved, with related clinical trials reaching 1540, and as many as 2304 patents.
Daxibotulinumtoxin targets SNAP25 and is indicated for the treatment of nervous system diseases, skin, and musculoskeletal diseases. The drug has received approval in the United States and is currently undergoing the regulatory process in China. Its first approval date globally was in September 2022, and it has been designated as an orphan drug.