Pharmaceutical Insights: Nemolizumab's R&D Progress and its Mechanism of Action on Drug Target
Nemolizumab's R&D Progress
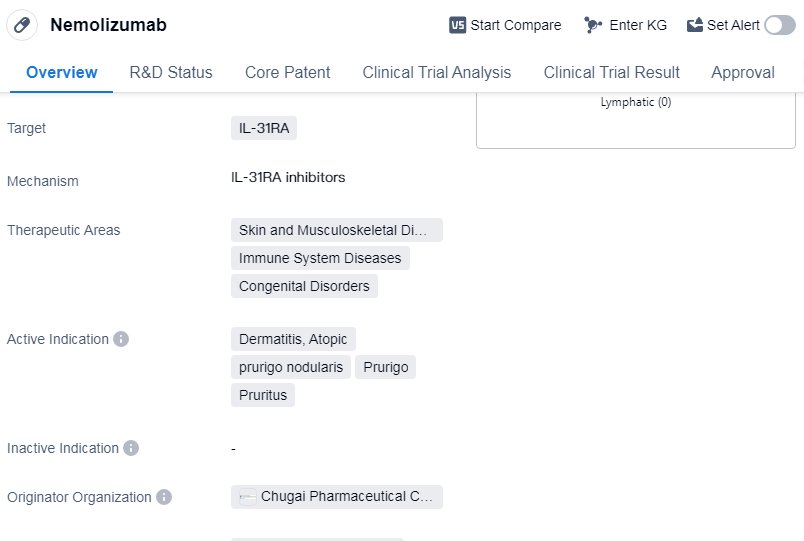
Nemolizumab is a monoclonal antibody drug that targets IL-31RA, making it a promising treatment option for various conditions related to skin and musculoskeletal diseases, immune system diseases, and congenital disorders. The drug has received its highest phase of approval, indicating its potential efficacy and safety.
The active indications for Nemolizumab include dermatitis, atopic, prurigo nodularis, prurigo, and pruritus. These conditions are characterized by skin inflammation, severe itching, and discomfort, which can significantly impact the quality of life for patients. By targeting IL-31RA, Nemolizumab aims to alleviate these symptoms and provide relief to affected individuals.
Chugai Pharmaceutical Co., Ltd. is the originator organization behind Nemolizumab. As a reputable pharmaceutical company, Chugai has developed this drug with the intention of addressing unmet medical needs in the field of biomedicine. Their expertise and dedication to research and development have led to the successful approval of Nemolizumab.
The first approval of Nemolizumab was granted in Japan in March 2022. This signifies that the drug has met the necessary regulatory requirements and has been deemed safe and effective for use in patients. The breakthrough therapy designation further highlights the potential of Nemolizumab to provide significant clinical benefits compared to existing treatment options.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nemolizumab: IL-31RA inhibitors
IL-31RA inhibitors are a type of drugs that target and inhibit the activity of the IL-31RA receptor. IL-31RA stands for Interleukin-31 receptor A, which is a protein found on the surface of certain cells in the body, including immune cells and skin cells. IL-31RA plays a role in the signaling pathway of interleukin-31 (IL-31), a cytokine that is involved in inflammation and immune responses.
By inhibiting IL-31RA, these inhibitors can block the binding of IL-31 to its receptor, thereby reducing the downstream effects of IL-31 signaling. IL-31 is known to be associated with various inflammatory conditions, particularly those affecting the skin, such as atopic dermatitis and pruritus (itching). Therefore, IL-31RA inhibitors have the potential to be used as therapeutic agents for these conditions.
From a biomedical perspective, IL-31RA inhibitors are being studied and developed as a targeted treatment approach for inflammatory skin disorders. By specifically targeting the IL-31RA receptor, these inhibitors aim to modulate the immune response and alleviate symptoms associated with IL-31-mediated inflammation. Clinical trials and research are ongoing to evaluate the safety and efficacy of IL-31RA inhibitors in the management of these conditions.
Drug Target R&D Trends for Nemolizumab
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 1 IL-31RA drugs worldwide, from 4 organizations, covering 4 indications, and conducting 40 clinical trials.
The analysis of target IL-31RA reveals a competitive landscape with multiple companies actively involved in its development. Maruho Co., Ltd., Nuvisan GmbH, Galderma Holding SA, and Roche Holding AG are among the companies showing significant growth and R&D progress. The approved indication of dermatitis, atopic highlights the efficacy of IL-31RA drugs in treating this condition. Monoclonal antibodies are the most rapidly progressing drug type under IL-31RA, indicating their potential in this therapeutic area. The development of IL-31RA drugs is widespread across various countries/locations, indicating global interest and potential market expansion. Further research and development efforts are expected to shape the future development of IL-31RA and its applications in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Nemolizumab represents a significant advancement in the field of biomedicine, particularly in the treatment of skin and musculoskeletal diseases, immune system diseases, and congenital disorders. Its monoclonal antibody nature and specific targeting of IL-31RA make it a promising therapeutic option for patients suffering from dermatitis, atopic, prurigo nodularis, prurigo, and pruritus. With its recent approval in Japan, Nemolizumab has the potential to improve the lives of individuals affected by these conditions and pave the way for further advancements in the pharmaceutical industry.