Pharmaceutical Insights: Sulfamethazine's R&D Progress and its Mechanism of Action on Drug Target
Sulfamethazine's R&D Progress
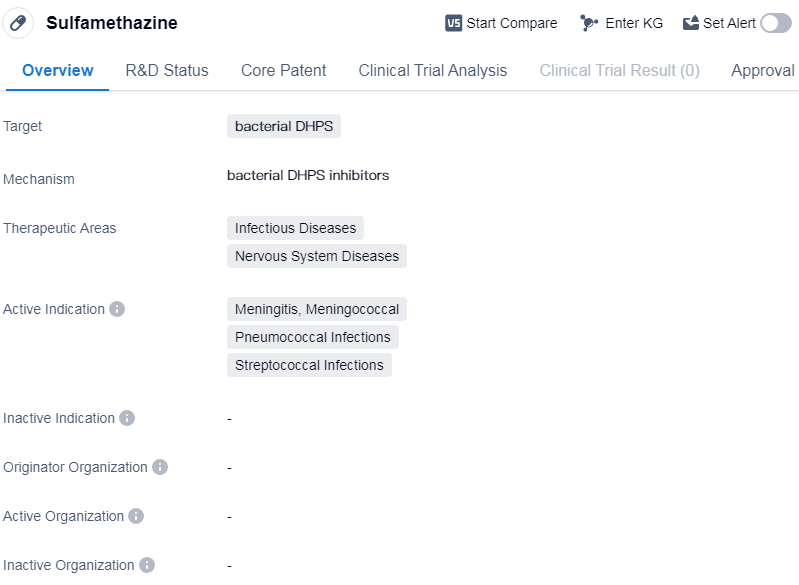
Sulfamethazine is a small molecule drug that primarily targets bacterial dihydropteroate synthase (DHPS). It falls under the therapeutic areas of infectious diseases and nervous system diseases. The drug is indicated for the treatment of various infections, including meningitis (specifically meningococcal and pneumococcal infections) as well as streptococcal infections.
Sulfamethazine has successfully completed the highest phase of clinical trials and has received approval globally. The drug was first approved in the United States in August 1949, making it a well-established medication with a long history of use.
As a small molecule drug, sulfamethazine is designed to target bacterial DHPS, an enzyme involved in the synthesis of folic acid in bacteria. By inhibiting this enzyme, sulfamethazine disrupts the growth and reproduction of bacteria, ultimately leading to their elimination.
The therapeutic areas of infectious diseases and nervous system diseases highlight the broad spectrum of conditions that sulfamethazine can potentially address. Infectious diseases encompass a wide range of bacterial infections, including those affecting the central nervous system, such as meningitis. Streptococcal infections, caused by the bacteria streptococcus, are also among the active indications for sulfamethazine.
The fact that sulfamethazine has reached the highest phase of clinical trials and received approval in global markets indicates its efficacy and safety profile. Its first approval in the United States in 1949 further emphasizes its long-standing presence in the pharmaceutical market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sulfamethazine: Bacterial DHPS inhibitors
Bacterial DHPS inhibitors are a type of drugs that specifically target and inhibit the enzyme dihydropteroate synthase (DHPS) in bacteria. DHPS is an essential enzyme involved in the synthesis of folate, which is necessary for the production of DNA and other important molecules in bacteria. By inhibiting DHPS, these inhibitors disrupt the bacterial folate synthesis pathway, leading to the inhibition of bacterial growth and reproduction.
From a biomedical perspective, bacterial DHPS inhibitors are important in the field of antimicrobial therapy. They are specifically designed to target bacterial DHPS enzymes, making them effective against bacterial infections. By inhibiting the synthesis of folate, these inhibitors disrupt the essential processes required for bacterial survival and replication. This targeted approach helps to minimize the risk of toxicity to human cells, as human cells do not possess DHPS enzymes and rely on dietary folate intake instead.
Bacterial DHPS inhibitors are commonly used in the treatment of bacterial infections caused by pathogens such as Escherichia coli, Staphylococcus aureus, and Streptococcus pneumoniae. They are often used in combination with other antibiotics to enhance their efficacy and prevent the development of drug resistance. Examples of bacterial DHPS inhibitors include sulfonamides and trimethoprim, which are frequently prescribed for urinary tract infections, respiratory tract infections, and other bacterial infections.
Overall, bacterial DHPS inhibitors play a crucial role in combating bacterial infections by selectively targeting an essential enzyme in bacteria, thereby disrupting their growth and survival.
Drug Target R&D Trends for Sulfamethazine
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 20 bacterial DHPS drugs worldwide, from 17 organizations, covering 18 indications, and conducting 120 clinical trials.
Based on the analysis of the provided data, Pfizer Inc. is the leading company in the development of drugs targeting bacterial DHPS, with the highest number of drugs in the approved phase. The most common indication for these drugs is bacterial infections. Small molecule drugs are progressing rapidly in the development of treatments for bacterial DHPS. China is the country with the fastest development in this field, with the highest number of drugs in the approved phase. The competitive landscape for target bacterial DHPS is diverse, with multiple companies and countries actively involved in research and development. The future development of target bacterial DHPS holds promise for the treatment of various bacterial infections and other related conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sulfamethazine is a small molecule drug that targets bacterial DHPS and is indicated for the treatment of various infectious diseases, including meningitis and streptococcal infections. It has successfully completed clinical trials and received approval globally. With its first approval dating back to 1949 in the United States, sulfamethazine has a well-established history in the pharmaceutical industry.