Pharmaceutical Insights: Zoledronic Acid's R&D Progress and its Mechanism of Action on Drug Target
Zoledronic Acid's R&D Progress
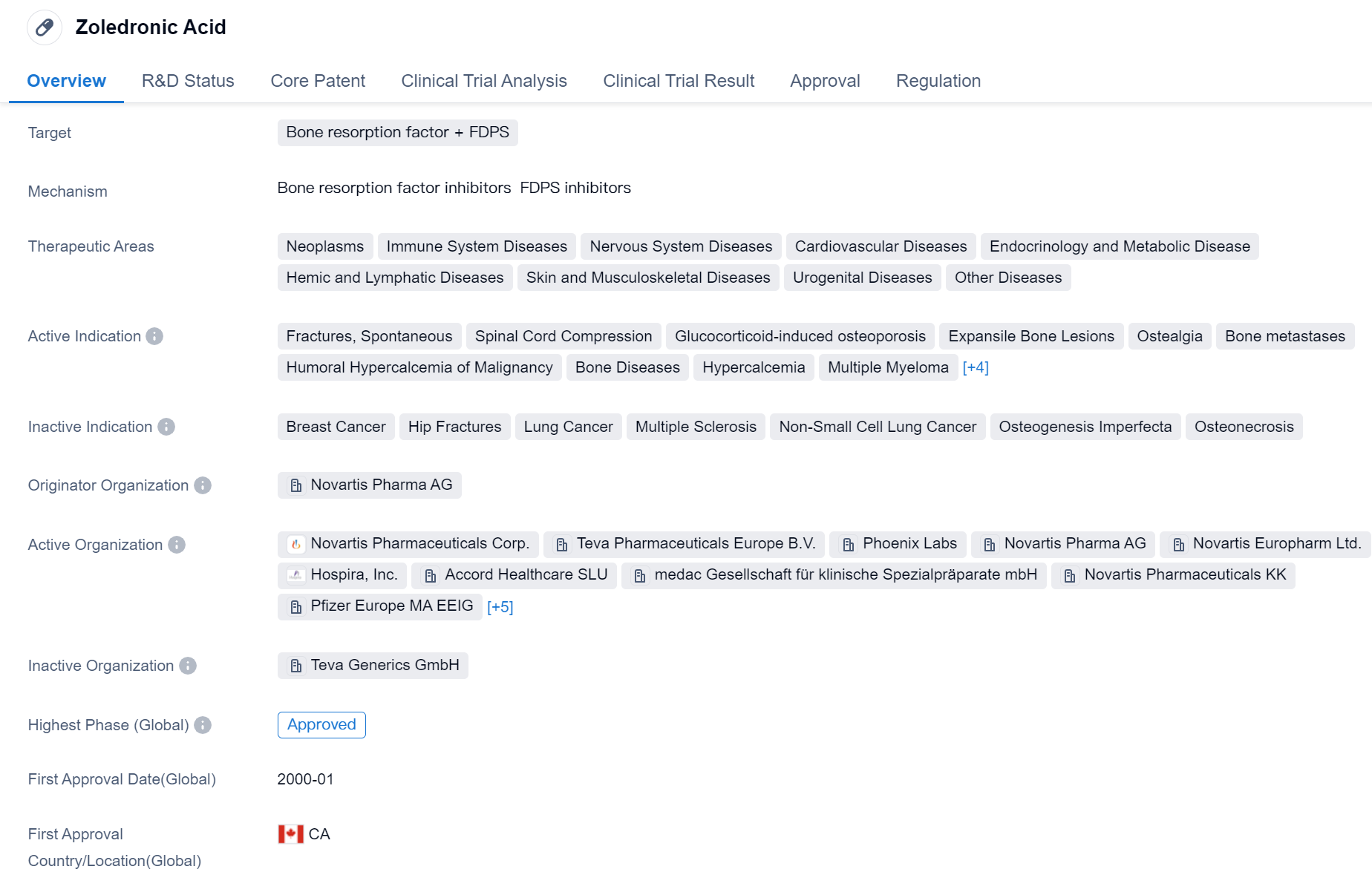
Zoledronic Acid is a small molecule drug that targets bone resorption factor and FDPS. It has been approved for various therapeutic areas including neoplasms, immune system diseases, nervous system diseases, cardiovascular diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases. The drug is indicated for the treatment of fractures, spontaneous spinal cord compression, glucocorticoid-induced osteoporosis, expansile bone lesions, ostealgia, bone metastases, humoral hypercalcemia of malignancy, bone diseases, hypercalcemia, multiple myeloma, osteitis deformans, osteoporosis, osteoporosis postmenopausal, and prostatic cancer.
Zoledronic Acid was developed by Novartis Pharma AG and received its first approval in Canada in January 2000. It has since been approved in various countries globally. The drug has undergone fast track regulation and has also been designated as an orphan drug.
Zoledronic Acid is primarily used for the treatment of bone-related conditions such as fractures, osteoporosis, and bone metastases. It works by inhibiting bone resorption, which helps to prevent bone loss and reduce the risk of fractures. The drug has shown efficacy in reducing bone pain and improving bone density in patients with osteoporosis and multiple myeloma.
The approval of Zoledronic Acid in multiple therapeutic areas highlights its versatility and potential for treating a wide range of diseases. Its ability to target bone resorption factor and FDPS makes it a valuable option for patients with bone-related conditions. The drug's fast track designation and orphan drug status further emphasize its importance in addressing unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Zoledronic Acid: Bone resorption factor inhibitors FDPS inhibitors
Bone resorption factor inhibitors, also known as FDPS inhibitors, are a class of drugs that work by inhibiting the activity of the enzyme farnesyl diphosphate synthase (FDPS). FDPS is an essential enzyme involved in the mevalonate pathway, which is responsible for the production of isoprenoid compounds necessary for various cellular processes, including bone resorption.
In the context of biomedicine, bone resorption factor inhibitors are primarily used in the treatment of bone-related disorders such as osteoporosis and bone metastases. These inhibitors help to reduce excessive bone resorption by blocking the activity of FDPS, thereby preventing the breakdown of bone tissue. By inhibiting bone resorption, these drugs can help to maintain bone density and prevent bone loss.
Bone resorption factor inhibitors are typically administered orally or through injection, depending on the specific drug. They may be used as standalone treatments or in combination with other medications, such as hormone replacement therapy or bisphosphonates, to enhance their therapeutic effects.
It's important to note that the term "FDPS inhibitors" specifically refers to the mechanism of action of these drugs by targeting the FDPS enzyme. Other classes of drugs may also inhibit bone resorption through different mechanisms, such as RANKL inhibitors or bisphosphonates.
Drug Target R&D Trends for Zoledronic Acid
Bone resorption factor, also known as FDPS (farnesyl diphosphate synthase), plays a crucial role in the human body. It is an enzyme involved in the mevalonate pathway, responsible for the production of isoprenoids, essential molecules for various cellular functions. In bone metabolism, FDPS is particularly important as it regulates the resorption process, which is necessary for bone remodeling and maintenance. By controlling the production of isoprenoids, FDPS influences the activity of osteoclasts, the cells responsible for bone resorption. Understanding the role of FDPS in bone resorption is vital for developing therapeutic interventions to treat conditions such as osteoporosis and other bone-related disorders.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 4 Bone resorption factor + FDPS drugs worldwide, from 20 organizations, covering 27 indications, and conducting 565 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Zoledronic Acid is a small molecule drug developed by Novartis Pharma AG. It has been approved for various therapeutic areas and is indicated for the treatment of fractures, osteoporosis, bone metastases, and other bone-related conditions. The drug's approval in multiple countries, fast track regulation, and orphan drug designation highlight its significance in the pharmaceutical industry.