Phase III trial results indicate Xofluza greatly lowers the spread of flu viruses
Genentech, part of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), revealed favorable topline results from the Phase III CENTERSTONE trial of Xofluza® (baloxavir marboxil), an antiviral medication, indicating a decrease in influenza virus transmission. The trial achieved its primary goal, showing that a single oral dose of Xofluza administered to individuals with influenza substantially lowered the chances of their household members getting infected. Xofluza was well tolerated, with no new safety concerns discovered.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
CENTERSTONE is the first global Phase III trial demonstrating a reduction in transmission with an antiviral treatment for a respiratory viral illness. This new information could enhance the benefits of Xofluza, which is already approved for treating symptoms and preventing infection post-virus exposure. The topline results will be unveiled at the 2024 OPTIONS XII for the Control of Influenza Congress, scheduled for September 29 – October 2 in Brisbane, Australia.
"This new finding on transmission reduction builds on Xofluza’s proven effectiveness in treating and preventing influenza after exposure, signifying a crucial advancement that could enhance health outcomes both at individual and community levels," said Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. "We anticipate discussions with regulatory bodies and public health organizations regarding these data for better influenza pandemic preparedness, aiming to deliver these benefits to patients."
Influenza is a prevalent but serious infectious disease, imposing a significant public health burden. Since 2010, seasonal influenza in the U.S. has infected up to 41 million individuals annually, leading to thousands of hospitalizations and 51,000 deaths each year. Given the concurrent circulation of multiple respiratory viruses (including COVID-19) impacting people throughout and beyond the winter season, it is crucial to not underestimate influenza. Early diagnosis and treatment are essential for the effective management of both seasonal and pandemic influenza.
The CENTERSTONE study has received partial support through federal funding from the U.S. Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under Other Transaction Agreement number: HHSO100201800036C.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
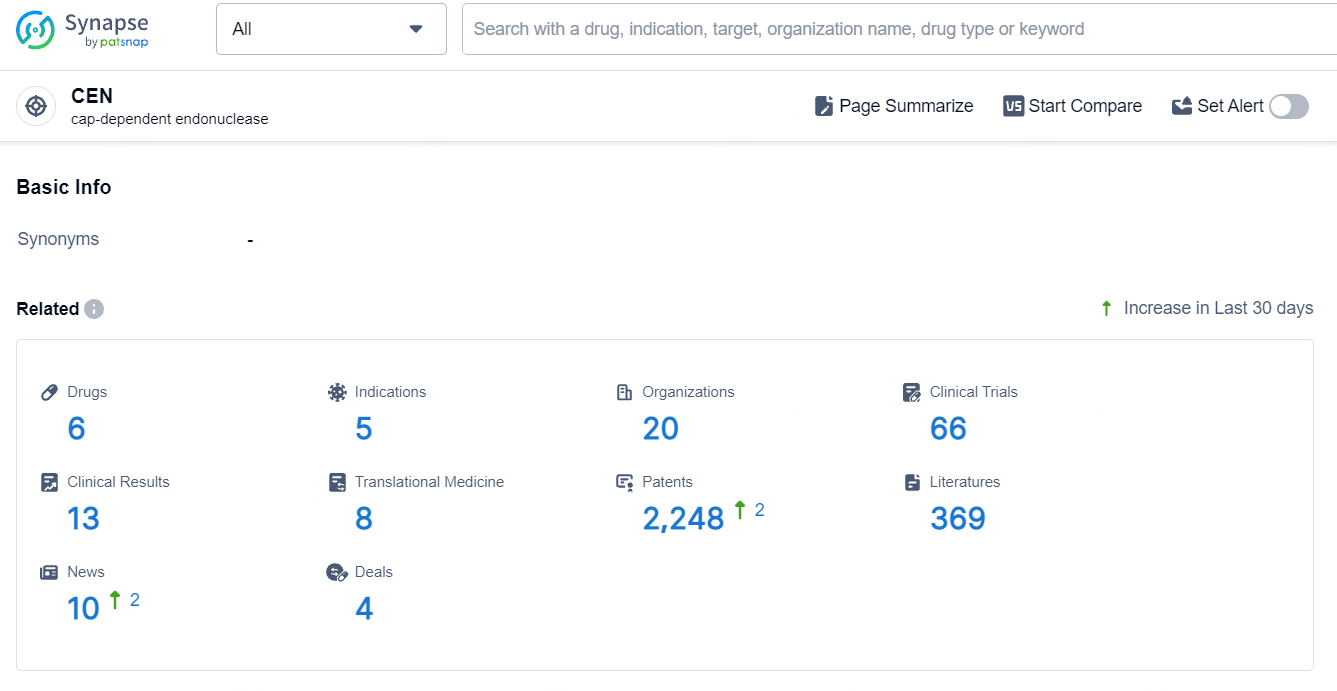
According to the data provided by the Synapse Database, As of September 23, 2024, there are 6 investigational drugs for the CEN target, including 5 indications, 20 R&D institutions involved, with related clinical trials reaching 66, and as many as 2248 patents.
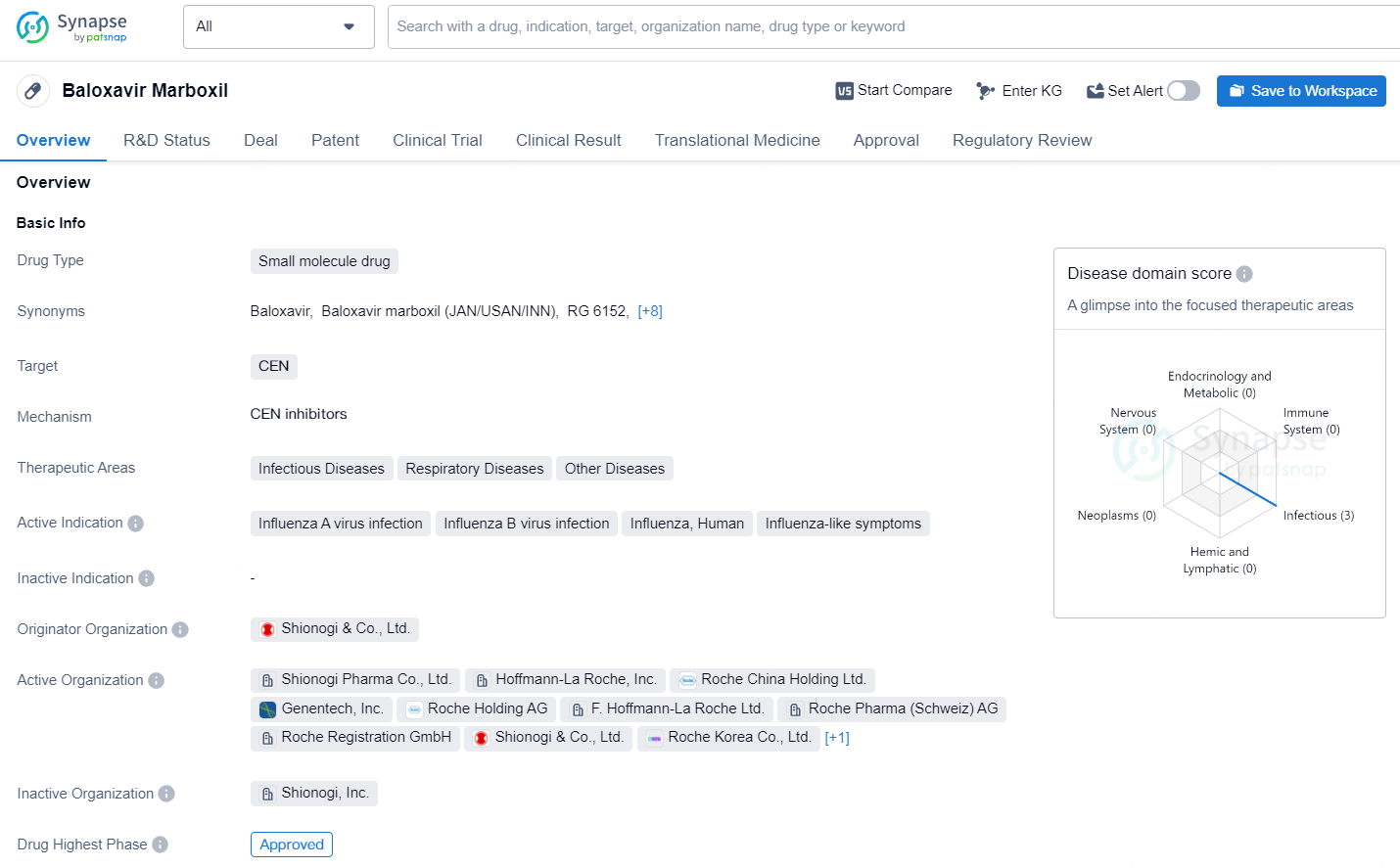
Baloxavir marboxil is a small molecule drug developed by Shionogi & Co., Ltd. It is primarily used to treat infectious diseases, respiratory diseases, and other related conditions. The drug targets the cap-dependent endonuclease (CEN) and is indicated for the treatment of influenza A and B virus infections, as well as influenza-like symptoms.