Polaris Group begins phased BLA submission for ADI-PEG 20 to the U.S. FDA for Malignant Pleural Mesothelioma management
The initiation of the rolling submission of Polaris Group's BLA for ADI-PEG 20 to the FDA has been declared by the company. This is in relation to the systematic care of patients who suffer from malignant pleural mesothelioma with non-epithelioid histology. The treatment combines the use of a platinum agent and pemetrexed.
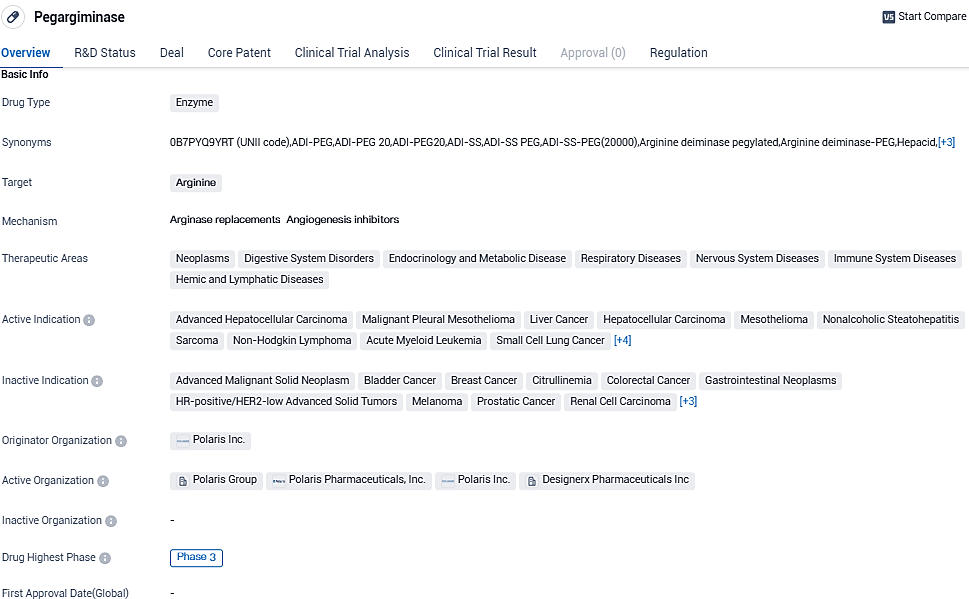
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This particular submission is informed by the successful phase 3 trial results for ADI-PEG 20, used together with a platinum agent and pemetrexed. It achieved its primary and secondary goals for progression-free and overall survival. This rolling submission methodology permits the submission of distinct parts of the BLA as soon as they're finished, therefore potentially speeding up the regulatory approval process.
The first submission to the FDA in the U.S comprises of the nonclinical and clinical segments of the BLA for ADI-PEG 20. It incorporates plans to round off the remaining chemistry, manufacturing, and controls components in the subsequent months. Once the final segment of the BLA is submitted, Polaris Group aims to acquire priority review status for this BLA. This, if accepted, could fast-track the regulatory approval procedure.
Dr. John Bomalaski, Polaris Group's Executive Vice President of Medical Affairs, said, "Our clinical trials have shown positive results, with ADI-PEG 20 presenting a substantial possibility to meet patients' medical requirements with malignant pleural mesothelioma. Our extensive development scheme is set up to provide solid data that encourages the FDA's review of our BLA."
Further, Howard Chen, CEO, and Chairman of Polaris Group noted, "We’re totally devoted to addressing the dire medical needs of patients diagnosed with malignant pleural mesothelioma. Our current submission gears us towards broadening the treatment alternatives available to MPM-affected patients in the U.S. We welcome the potential FDA approval as it indicates progress in our endeavor to manage hard-to-treat cancers. We anticipate a close collaboration with the FDA throughout their review operations."
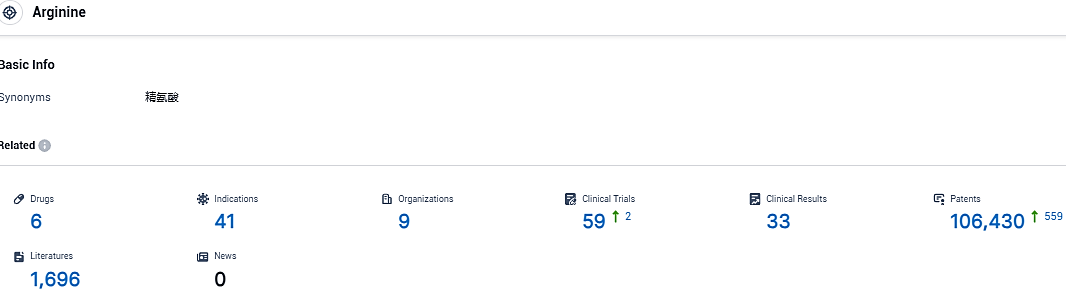
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 23, 2023, there are 6 investigational drugs for the arginine target, including 41 indications, 9 R&D institutions involved, with related clinical trials reaching 59, and as many as 106430 patents.
Pegargiminase is an enzyme-based drug that targets arginine. It shows potential for treating a wide range of diseases in various therapeutic areas, including neoplasms, digestive system disorders, and respiratory diseases. With its highest phase of development being Phase 3, Pegargiminase has reached an advanced stage of clinical evaluation. Its orphan drug status further emphasizes its potential to address unmet medical needs in specific patient populations.