Polpharma Biologics' Biosimilar Matches Entyvio® in Pharmacokinetics and Dynamics Study
Polpharma Biologics, a global biotechnology firm specializing in the creation and production of biosimilar drugs, has released preliminary findings that confirm the pharmacokinetic and pharmacodynamic similarities between its biosimilar product, PB016, and the original medication, Entyvio® (vedolizumab).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The study outcomes were derived from a single-administration, controlled, double-masked, 3-way parallel comparison clinical trial designed to evaluate the pharmacokinetics/pharmacodynamics (PK/PD) and immune response of PB016 in relation to Entyvio® following intravenous infusion in a group of 120 healthy participants. PB016 demonstrated equivalence to Entyvio® across all measured PK and PD indicators, while presenting no concerns regarding immune response or safety when compared to the original medication.
"These findings support its role as a cost-effective alternative for the approximately 3.5 million individuals affected by inflammatory bowel disease across Europe and the USA. With its leading position in the biosimilars space, PB016 aims at a sector generating over $5.3 billion from annual sales of Entyvio®, projected to rise beyond $7 billion by the year 2030," Konstantin Matentzoglu, a representative of the Supervisory Board as well as the executive management at Polpharma Biologics Group, stated.
"Having already established partnerships with esteemed firms for two biosimilars in our pipeline, it's with a sense of pride that we announce PB016 advancing towards the final phases of development under our brand. Our experts have meticulously crafted this therapy, from generating the initial cell line to scaling up production capabilities entirely within our facilities, paving the way for securing lucrative future alliances for this product," Matentzoglu further commented.
As part of its ongoing efforts to substantiate PB016's effectiveness and safety, alongside its immune response profile compared with the original medication, Polpharma Biologics is actively engaged in an extensive global clinical study focusing on patients with ulcerative colitis.
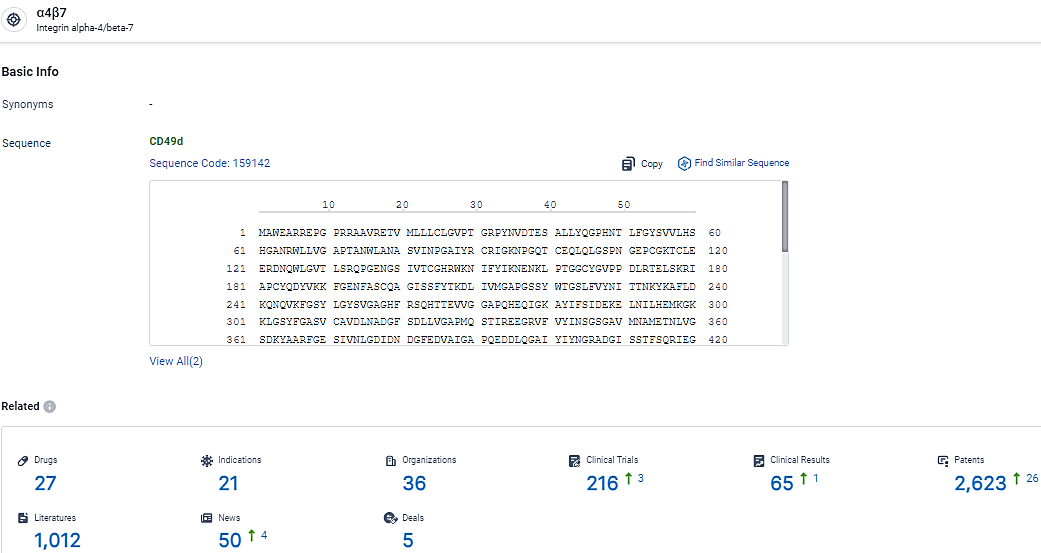
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 26, 2024, there are 27 investigational drugs for the α4β7 target, including 21 indications,36 R&D institutions involved, with related clinical trials reaching 216, and as many as 2623 patents.
Vedolizumab is a monoclonal antibody that targets the α4β7 integrin, a protein found on gut homing T helper lymphocytes, reducing gastrointestinal inflammation. Vedolizumab is approved in over 70 countries worldwide, including Japan, Canada, and Australia, though its approved uses may vary. These chronic bowel conditions affect over 3.5 million people in Europe and the US, often significantly impacting patients' daily functioning and quality of life. Factors like genetics and increasing urbanization may be contributing to growing prevalence of these diseases.