Voyager's Gene Therapy Shows Promise in Early Alzheimer's Trial, Advances to Later Phase
Voyager Therapeutics, Inc., has unveiled novel findings from their dual preclinical studies directed at combating abnormal tau proteins as a therapy for Alzheimer's disease. Details regarding VY-TAU01, the company's prime candidate in the anti-tau antibody initiative, alongside insights into their gene therapy endeavor aimed at gene silencing of tau, are scheduled to be introduced at the forthcoming International Conference on Alzheimer's and Parkinson's Diseases and related neurodegenerative conditions. This event is set to occur between the 5th and 9th of March 2024, in the city of Lisbon, Portugal.
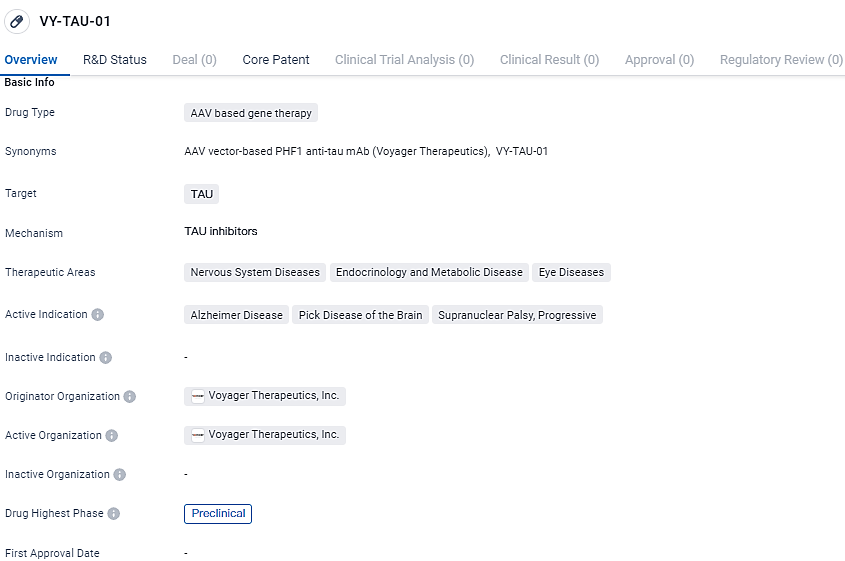
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Research findings indicate that a solitary dose of Voyager's experimental gene therapy aimed at tau silencing, when injected intravenously into mice engineered to produce human tau, facilitated widespread dispersal of the AAV vector within various cerebral regions. This occurred alongside a proportionate decline in tau messenger RNA, with reductions reaching up to 90%. These reductions correlated with substantial decreases in the human tau protein throughout the mice's brains.
The therapeutic initiative from Voyager converges vectorized siRNA aimed at tau with an innovative capsid that can traverse the blood-brain barrier (BBB). This capsid has origins in the company's distinct TRACER™ discovery platform. Leveraging these insights, Voyager is propelling this venture into a more advanced research phase and is on track to submit a proposal for an investigational new drug (IND) in the year 2026.
Additionally, Voyager is poised to unveil novel preclinical figures showing that VY-TAU01—its foremost antibody pointed at pathological tau—and Ab-01, the murine equivalent, displayed a positive safety profile and exhibited promising pharmacokinetic characteristics post intravenous infusion in both non-human primates and P301S mutant mice featuring pathological human tau. With these developments, the filing of IND for VY-TAU01 is foreseen for the first semester of 2024.
"The team at Voyager is increasingly optimistic about the role of modalities focusing on pathological tau in enhancing the clinical outlook for those afflicted by Alzheimer’s disease," commented Todd Carter, Ph.D., the Chief Scientific Officer at Voyager Therapeutics.
Dr. Carter went on to explicate, "Novel data from external sources involving investigational treatments that target tau have shown a reduction in the propagation of pathological tau, as detected through tau PET scans, alongside encouraging cognitive performance indicators. Our team is eager to keep pushing forward our dual efforts trained on tau as well as to monitor forthcoming results from companion research entities, which we believe will be significant in corroborating the efficacy of this therapeutic target."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
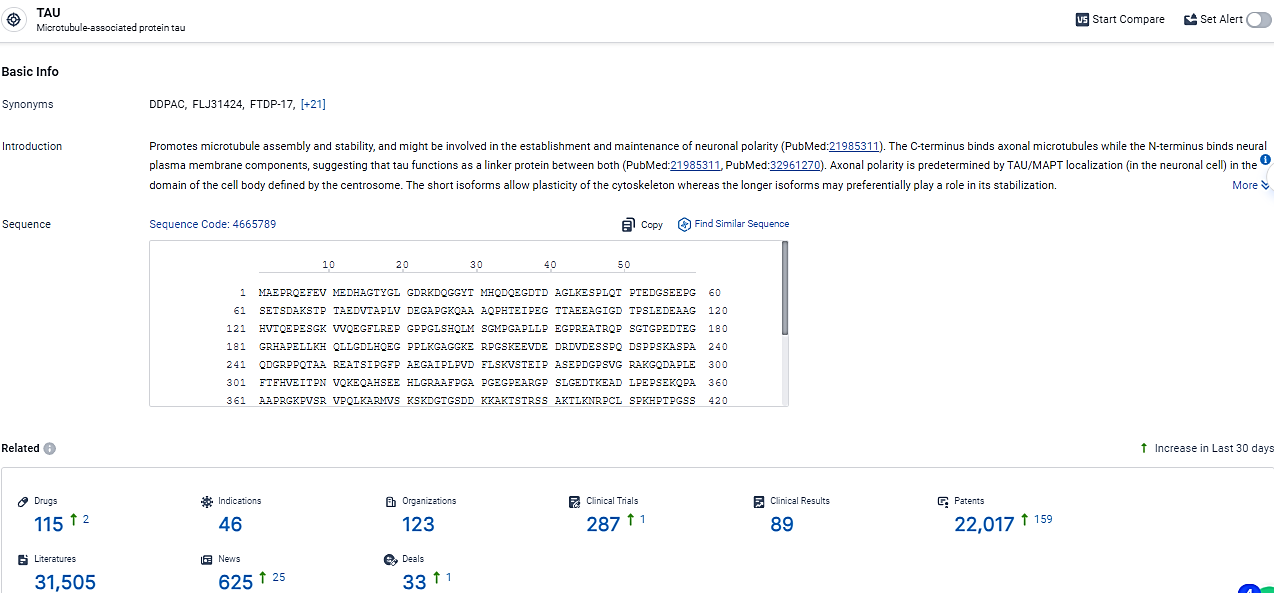 According to the data provided by the Synapse Database, As of February 26, 2024, there are 115 investigational drugs for the TAU target, including 46 indications, 123 R&D institutions involved, with related clinical trials reaching 287, and as many as 22017 patents.
According to the data provided by the Synapse Database, As of February 26, 2024, there are 115 investigational drugs for the TAU target, including 46 indications, 123 R&D institutions involved, with related clinical trials reaching 287, and as many as 22017 patents.
VY-TAU-01 targets TAU, a protein involved in various nervous system diseases. The drug aims to address neurodegenerative disorders such as Alzheimer's disease, Pick disease of the brain, supranuclear palsy, and progressive supranuclear palsy. Currently, VY-TAU-01 is in the preclinical phase of development, and further studies are needed to determine its safety and efficacy in humans.