Preliminary MTX-101 Data for Autoimmune Management Unveiled by Mozart Therapeutics at 2024 SOT Meeting
Mozart Therapeutics, a pioneering company at the forefront of creating therapies that utilize modulators of CD8 Treg cells in combating autoimmune and inflammatory conditions, has declared the release of novel preclinical findings concerning their compound MTX-101. This information is set to be unveiled through a newly added poster session at the 63rd yearly gathering of the Society of Toxicology, which will occur from March 10th to 14th in the city of Salt Lake, Utah.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
MTX-101 constitutes a dual-affinity treatment agent for autoimmune regulation, specifically engaging with CD8 T regulatory cells through its antibody composition. Due to the observed minimal cross-reactivity in animal studies when identifying its targets, researchers employed a model involving IL-15 humanized transgenic mice for preclinical evaluations of both toxicity and drug actions.
"It's encouraging to share preliminary findings that confirm the preclinical tolerability of MTX-101. Our investigations emphasize the effectiveness of utilizing the IL-15 humanized transgenic mouse model to examine the interaction with human immune receptors, minimally affected by cross-reactivity in standard toxicological test species," asserted Dr. Kristine Swiderek, the lead scientific strategist at Mozart Therapeutics. "This particular model is instrumental for determining the safety profile before clinical trials and comprehending the pharmacokinetic and pharmacodynamic interactions that will help shape our strategy for advancing MTX-101 through clinical stages."
As an innovative immunotherapeutic agent, MTX-101 targets the CD8 T cell subpopulation involved in regulatory functions by binding to specific markers, KIR and CD8. The intention behind this inhibitor is to rejuvenate the natural regulatory role of CD8 T cells at the early stages of an autoimmune condition, with the goal of suppressing the harmful autoimmune cells, mitigating subsequent inflammatory responses, and averting damage to bodily tissues.
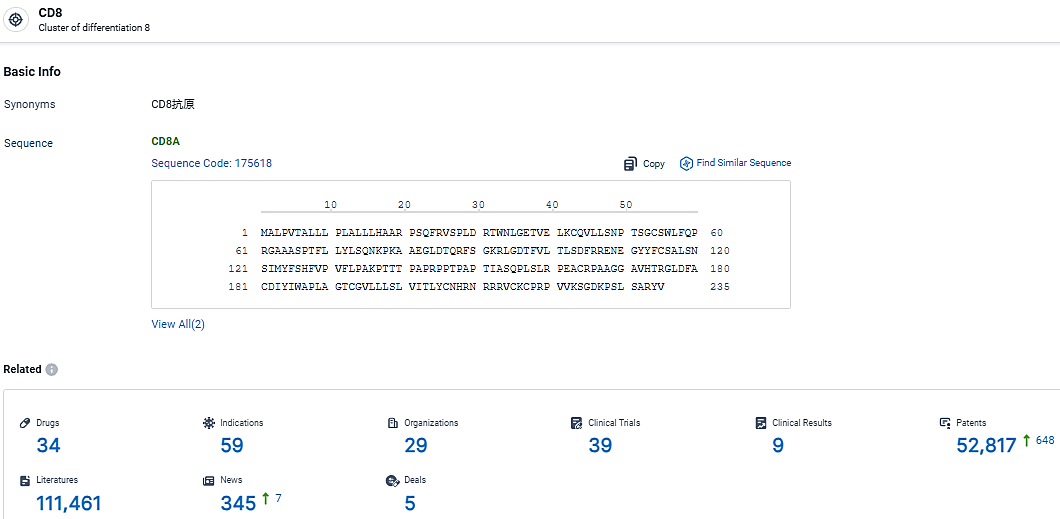
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 17, 2024, there are 34 investigational drugs for the CD8 target, including 59 indications, 29 R&D institutions involved, with related clinical trials reaching 39, and as many as 52817 patents.
MTX-101 targets CD8 and aims to provide therapeutic benefits for gastrointestinal diseases. As of now, it is in the preclinical phase, indicating that further research and testing are required before it can advance to human clinical trials.