Promising Early Results for ACELYRIN's Lonigutamab in Thyroid Eye Disease Studies
ACELYRIN, INC., a biopharmaceutical entity engaged in the latter phases of clinical research, is devoted to hastening the creation and distribution of innovative therapeutic solutions targeting immunological conditions. The company recently publicized encouraging preliminary results from a continuing Phase 1/2 study concerning the investigational medical compound lonigutamab for the treatment of thyroid eye disease.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Administered via the subcutaneous route, Lonigutamab is a humanized monoclonal antibody of the IgG1 class, specifically devised to engage the insulin-like growth factor-1 receptor (IGF-1R)—a recognized target for intervention in thyroid eye disease (TED).
During its early-phase clinical trial, spanning Phase 1 and 2, Lonigutamab exhibited prompt effects on eye bulging, otherwise known as proptosis, and the clinical activity score, with notable changes observed as early as three weeks following the initial dose.
"The pursuit of a remedy for thyroid eye disease holds profound significance to our team. We extend our gratitude to both the participants and the research personnel collaborating with us. With the data acquired, we are optimistic about Lonigutamab's potential in treating TED and are actively progressing its clinical development towards patient improvement," expressed Shao-Lee Lin, MD, PhD, the guiding force and chief executive at ACELYRIN.
Within this comprehensive, multi-institutional Phase 1/2 study, investigators are examining Lonigutamab's therapeutic profile in individuals with TED, focusing on both its safety and effectiveness. Initial testing involved a controlled setup where Lonigutamab was dispensed to six participants and a placebo to two. The subsequent cohort received only Lonigutamab in an open-label scheme, with preliminary findings from six individuals at the six-week mark.
To date, the clinical exposures to Lonigutamab have revealed a favorable tolerability with no severe adverse effects, hyperglycemia, or hearing deficits reported.
Shoaib Ugradar, MD, belonging to the Orbital and Oculoplastic Surgery sector in Beverly Hills, responded optimistically to these developments: "The positive outcomes following subcutaneous Lonigutamab administration for anti-IGF-1R intervention are promising. Early patient response signals, within the span of three weeks after injection, indicate a substantial therapeutic potential. Moreover, the preliminary safety observations appear superior relative to existing treatment benchmarks."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 22, 2024, there are 96 investigational drugs for the IGF-1R target, including 203 indications, 125 R&D institutions involved, with related clinical trials reaching 441, and as many as 364 patents.
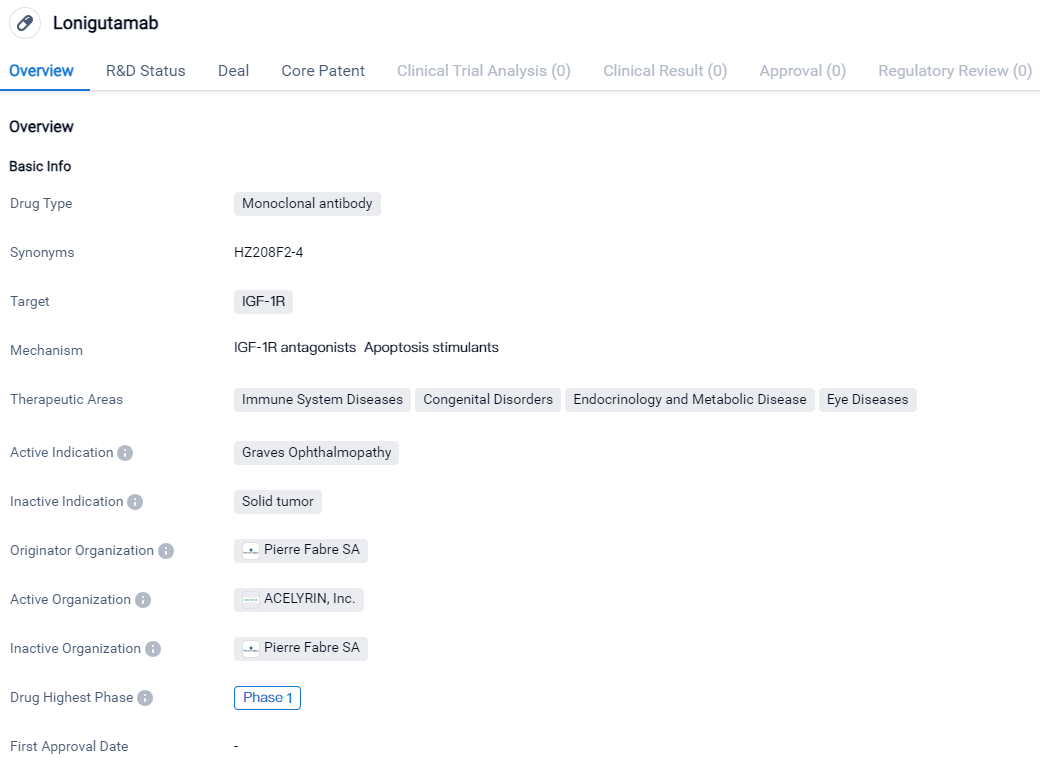
Lonigutamab is a monoclonal antibody drug that targets the IGF-1R receptor. It is currently in Phase 1 of development and shows potential in treating Graves Ophthalmopathy. The drug's therapeutic areas include immune system diseases, congenital disorders, endocrinology and metabolic diseases, as well as eye diseases. Lonigutamab represents a promising advancement in the pharmaceutical industry's efforts to address unmet medical needs in these areas.