23andMe Initiates Clinical Trial of Dual-Targeting Antibody 23ME-01473 Against ULBP6
23andMe Holding Co., an eminent enterprise specializing in human genetics and biopharmaceuticals, has confirmed the commencement of its Phase 1 clinical trial with the initial patient having received a dose of 23ME-01473. This investigational study focuses on treating individuals with advanced-stage solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The latest antibody being examined, ULBP6, was pinpointed utilizing 23andMe's exclusive research infrastructure, which is acknowledged as the planet's most extensive, recontactable, anonymous compendium of human genetic and phenotypic details. This marks the third therapeutic target to be genomically corroborated by 23andMe's platform and to commence clinical trials in less than four years.
"The advancement of this promising new dual-action NK-cell stimulator into human trials underscores the prowess of the 23andMe Therapeutics team and the transformative potential of our genetic research framework to pioneer and cultivate novel treatments based on human genetic insights," announced Jennifer Low, Director of Therapeutics Development at 23andMe. "We are enthusiastic about progressing with our investigation of ‘1473 and profoundly appreciate the individuals who are part of this clinical study."
Compound 23ME-01473 is designed to home in on ULBP6, with the aim of revitalizing anti-cancer immunity by way of NK and T cells. ULBPs are ligands induced under duress on the facade of tumor cells that couple with their corresponding receptor, NKG2D, on NK and T cells. To elude detection by immune cells, cancer can discard these ULBP ligands from their surface, thereby acting as deterrents that suppress the immune response.
Interruption of the attachment of free-floating ULBP6 to NKG2D using ‘1473 might reinstate the immune cells' ability to recognize and eliminate cancerous cells. Moreover, ‘1473 is augmented with Fc-effector functionality, granting NK cells an added stratagem to trigger apoptosis in cancer cells expressing ULBP6.
The potential of ULBP6 as a viable target in cancer treatment was ascertained with the aid of the 23andMe immune-oncology genetic signature. This methodology, conceived by 23andMe, seeks to uncover genetic variations conducive to immune enhancement and concomitant diminishment of cancer susceptibility. By parsing genetic data, 23andMe can detect genes related to immunity that are likely influential in the realm of cancer biology.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
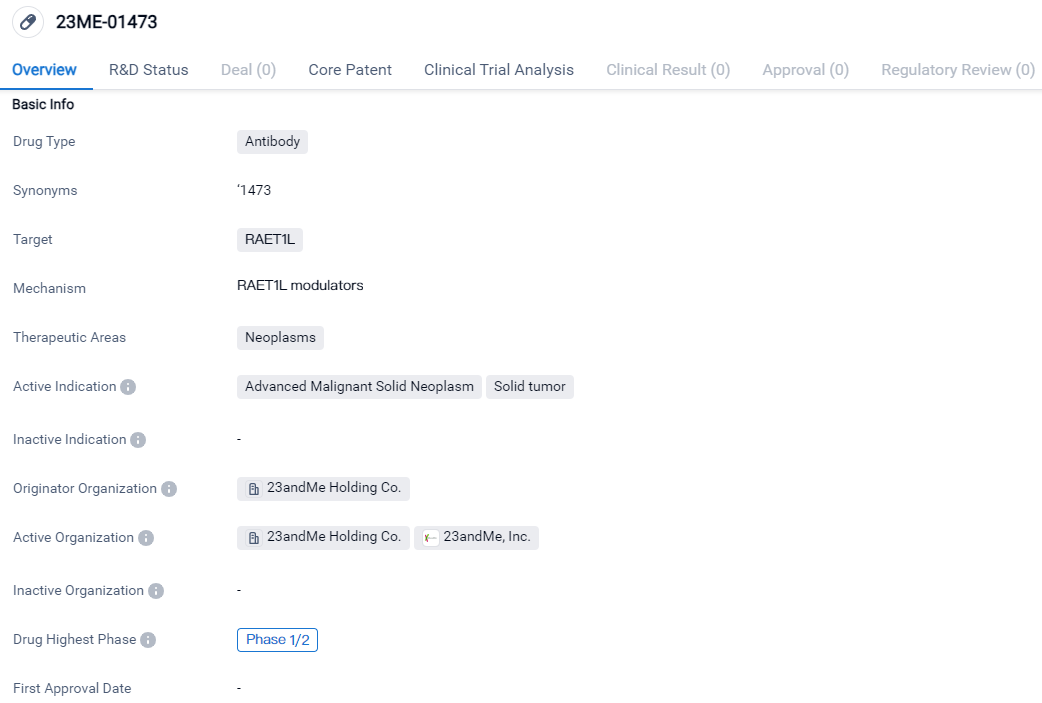
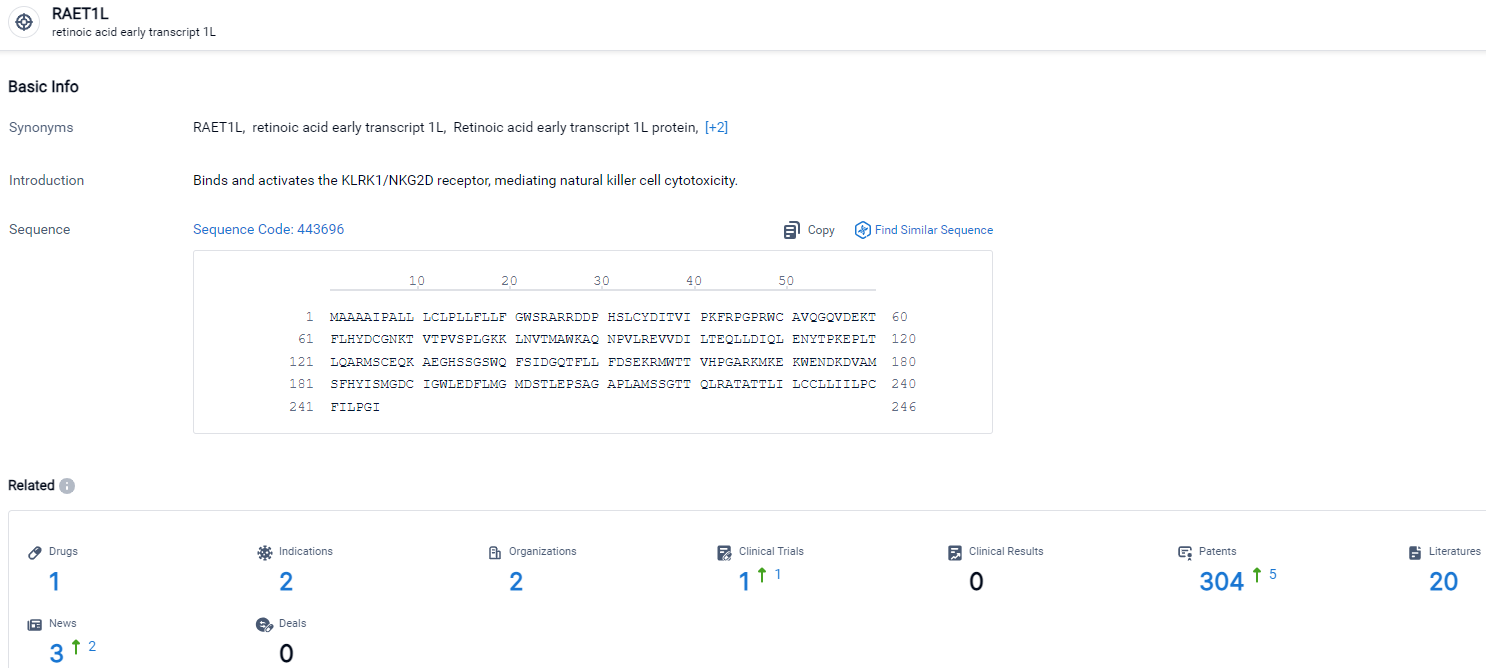
According to the data provided by the Synapse Database, As of March 22, 2024, there are 1 investigational drugs for the RAET1L target, including 2 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 304 patents.
23ME-01473 is an antibody that targets the protein RAET1L. It falls under the therapeutic area of neoplasms, specifically advanced malignant solid neoplasms and solid tumors. The drug is currently in the highest phase of development, which is Phase 1/2, on a global scale. The results of these trials will provide valuable insights into the drug's effectiveness and its potential to address the unmet medical needs in the field of neoplasms.